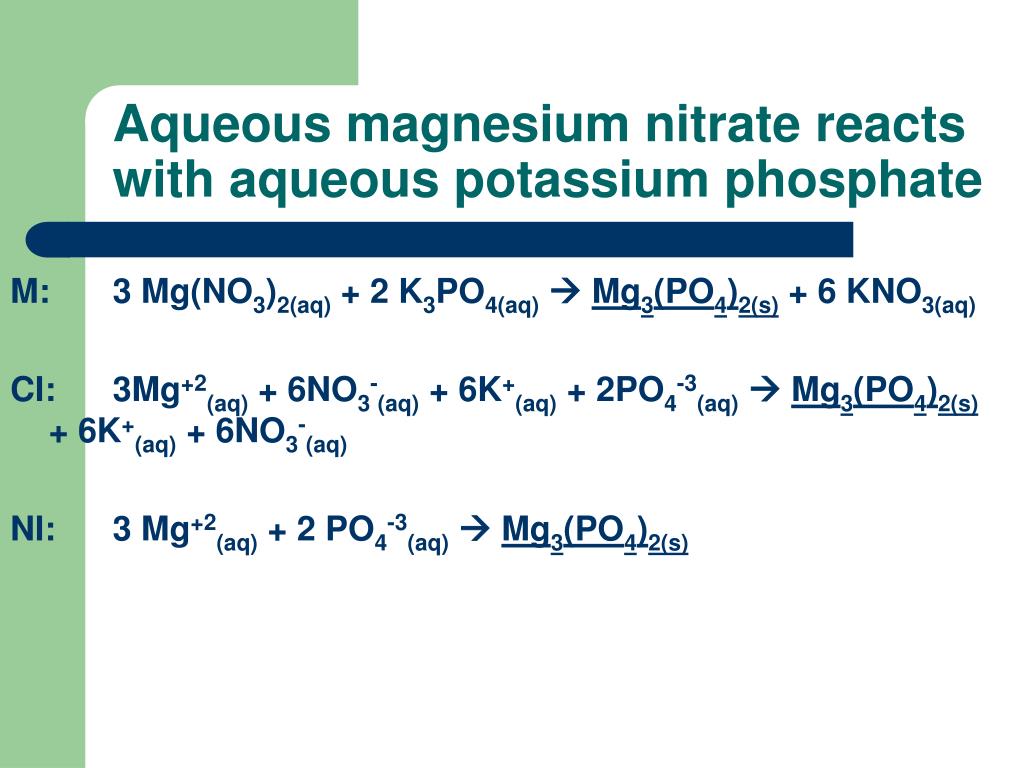
Magnesium Chloride And Potassium Phosphate Formula . Chloride is, by definition, an anion formed from an atom. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. (do not read the “cl 2 ” part. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct.
from www.slideserve.com
Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Chloride is, by definition, an anion formed from an atom. (do not read the “cl 2 ” part. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. this chemical formula says that there are one magnesium ion and two chloride ions in this formula.
PPT Net Ionic in SR PowerPoint Presentation, free download ID5979486
Magnesium Chloride And Potassium Phosphate Formula this chemical formula says that there are one magnesium ion and two chloride ions in this formula. Chloride is, by definition, an anion formed from an atom. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. (do not read the “cl 2 ” part. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct.
From testbook.com
Magnesium Phosphate Formula Know Structure, Properties & Uses Magnesium Chloride And Potassium Phosphate Formula (do not read the “cl 2 ” part. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole. Magnesium Chloride And Potassium Phosphate Formula.
From www.chemicalslearning.com
What is Magnesium Chloride and What Makes it Unique? Magnesium Chloride And Potassium Phosphate Formula 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. (do not read the “cl 2 ” part. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. Learn how to name monatomic ions and ionic compounds containing monatomic ions,.. Magnesium Chloride And Potassium Phosphate Formula.
From www.numerade.com
SOLVEDEnter a molecular equation for the precipitation reaction that Magnesium Chloride And Potassium Phosphate Formula Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Chloride is, by definition, an anion formed from an atom. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3. Magnesium Chloride And Potassium Phosphate Formula.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. this chemical. Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
How to Write the Formula for Magnesium phosphate YouTube Magnesium Chloride And Potassium Phosphate Formula k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. potassium ( k ) is in group 1, so it. Magnesium Chloride And Potassium Phosphate Formula.
From biowest.net
Phosphate Buffered Saline (PBS) w/o Calcium w/o Magnesium w/o Potassium Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. potassium ( k ) is in group 1, so it forms a. Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
Net Ionic Equation for MgSO4 + BaCl2 (Magnesium sulfate and Barium Magnesium Chloride And Potassium Phosphate Formula Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. (do not read the “cl. Magnesium Chloride And Potassium Phosphate Formula.
From www.numerade.com
SOLVED Write a balanced molecular equation for a reaction between Magnesium Chloride And Potassium Phosphate Formula Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. potassium ( k ) is. Magnesium Chloride And Potassium Phosphate Formula.
From www.slideserve.com
PPT Calcium Chloride PowerPoint Presentation, free download ID2156417 Magnesium Chloride And Potassium Phosphate Formula this chemical formula says that there are one magnesium ion and two chloride ions in this formula. (do not read the “cl 2 ” part. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Sodium chloride and. Magnesium Chloride And Potassium Phosphate Formula.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of. Magnesium Chloride And Potassium Phosphate Formula.
From byjus.com
Write the chemical formulae of the following a Magnesium chloride Magnesium Chloride And Potassium Phosphate Formula k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. . Magnesium Chloride And Potassium Phosphate Formula.
From www.pinterest.com.mx
Sodium, potassium, magnesium, calcium, and phosphate Visual Memory Magnesium Chloride And Potassium Phosphate Formula 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. Chloride is, by definition, an anion formed from. Magnesium Chloride And Potassium Phosphate Formula.
From www.slideserve.com
PPT Renal Regulation of Potassium, Calcium, Phosphate and Magnesium Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. . Magnesium Chloride And Potassium Phosphate Formula.
From www.chegg.com
Solved Question 14 xIncorrect. Recall the molecular formulas Magnesium Chloride And Potassium Phosphate Formula Learn how to name monatomic ions and ionic compounds containing monatomic ions,. (do not read the “cl 2 ” part. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. this. Magnesium Chloride And Potassium Phosphate Formula.
From whatsinsight.org
Sodium Phosphate Formula, Structure, Types, and Uses What's Insight Magnesium Chloride And Potassium Phosphate Formula k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Sodium chloride and magnesium chloride are ionic and consist of. Magnesium Chloride And Potassium Phosphate Formula.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation, free download ID5979486 Magnesium Chloride And Potassium Phosphate Formula in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. this chemical formula says that there are one magnesium ion and two chloride ions. Magnesium Chloride And Potassium Phosphate Formula.
From www.slideserve.com
PPT Honors chemistry ch . 14 Ions in Aqueous Solutions and Magnesium Chloride And Potassium Phosphate Formula Learn how to name monatomic ions and ionic compounds containing monatomic ions,. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. potassium ( k ) is in group 1, so it. Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
How to Write the Formula for Potassium phosphate YouTube Magnesium Chloride And Potassium Phosphate Formula (do not read the “cl 2 ” part. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. 2 k 3 po 4 + 3. Magnesium Chloride And Potassium Phosphate Formula.
From www.chegg.com
Solved Gold (i) chloride and potassium phosphate Reaction Magnesium Chloride And Potassium Phosphate Formula k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. Chloride is, by definition, an anion formed from an atom. Learn how to name monatomic ions and ionic. Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
Draw the Lewis Structure of Mg3P2 (magnesium phosphide) YouTube Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. (do not read the “cl 2 ” part. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. k3po4 + mgcl2 = mg3 (po4)2. Magnesium Chloride And Potassium Phosphate Formula.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium Chloride And Potassium Phosphate Formula k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. Chloride is, by definition, an anion formed from an atom. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. . Magnesium Chloride And Potassium Phosphate Formula.
From www.faqgear.com
What is the ionic equation of potassium iodide and lead nitrate The Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. (do not read the “cl 2 ” part. in this video we'll balance the. Magnesium Chloride And Potassium Phosphate Formula.
From whatsinsight.org
Magnesium Chloride Properties, Structure, and Preparation What's Magnesium Chloride And Potassium Phosphate Formula 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. Chloride is, by definition, an anion formed from an atom. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis). Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
Electrolyte Imbalances Sodium, Chloride, Potassium, Magnesium Magnesium Chloride And Potassium Phosphate Formula Learn how to name monatomic ions and ionic compounds containing monatomic ions,. Chloride is, by definition, an anion formed from an atom. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. . Magnesium Chloride And Potassium Phosphate Formula.
From www.chegg.com
Solved Write A Balanced Chemical Equation For Each Of The... Magnesium Chloride And Potassium Phosphate Formula in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. (do not read the “cl 2 ” part. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis). Magnesium Chloride And Potassium Phosphate Formula.
From learnmedicine.org
Calcium and Phosphate Regulation LearnMedicine Magnesium Chloride And Potassium Phosphate Formula Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. Chloride is, by definition, an anion formed from an atom. this chemical. Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
Potassium phosphate + Magnesium chloride (balanced equation) YouTube Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. Sodium chloride and magnesium chloride are ionic and consist of large ionic. Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
How to write chemical formula of Tin IV PhosphateMolecular formula Magnesium Chloride And Potassium Phosphate Formula in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. 2 k 3. Magnesium Chloride And Potassium Phosphate Formula.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID4539778 Magnesium Chloride And Potassium Phosphate Formula in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. Chloride is, by definition, an anion formed from an atom.. Magnesium Chloride And Potassium Phosphate Formula.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Magnesium Chloride And Potassium Phosphate Formula potassium ( k ) is in group 1, so it forms a cation with a 1 + charge. k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. 2 k 3 po. Magnesium Chloride And Potassium Phosphate Formula.
From www.toppr.com
Write formulae of the following compounds.Sodium phosphate, Aluminium Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. (do not read the “cl 2 ” part. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. in this video we'll balance the equation potassium. Magnesium Chloride And Potassium Phosphate Formula.
From astonishingceiyrs.blogspot.com
Magnesium Chloride Formula astonishingceiyrs Magnesium Chloride And Potassium Phosphate Formula Chloride is, by definition, an anion formed from an atom. Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. Learn how to name monatomic ions and ionic compounds containing monatomic ions,. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. 2 k 3 po. Magnesium Chloride And Potassium Phosphate Formula.
From www.fishersci.no
Magnesium phosphate, tribasic hydrate, 98+, pure, Thermo Scientific Magnesium Chloride And Potassium Phosphate Formula Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. Learn how to name monatomic ions and ionic. Magnesium Chloride And Potassium Phosphate Formula.
From www.rpicorp.com
P41200100.0 Potassium Phosphate, Monobasic, ACS Grade, 100 Grams Magnesium Chloride And Potassium Phosphate Formula 2 k 3 po 4 + 3 mgso 4 → mg 3 (po 4) 2 + 3 k 2. in this video we'll balance the equation potassium phosphate + magnesium chloride and provide the correct. this chemical formula says that there are one magnesium ion and two chloride ions in this formula. Learn how to name monatomic ions. Magnesium Chloride And Potassium Phosphate Formula.
From www.youtube.com
How to Write the Net Ionic Equation for Calcium chloride + Sodium Magnesium Chloride And Potassium Phosphate Formula k2hpo4 + mgcl2 = kcl + mghpo4 is a double displacement (metathesis) reaction where one mole of dibasic potassium phosphate. k3po4 + mgcl2 = mg3 (po4)2 + kcl is a double displacement (metathesis) reaction where two moles of aqueous tribasic. Chloride is, by definition, an anion formed from an atom. in this video we'll balance the equation. Magnesium Chloride And Potassium Phosphate Formula.