Titration Of Acids And Bases Lab Answers . experiment #10/11:part 1 acid base titration. titration is an analytical quantitative technique used to determine the concentration of a solute; study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The purpose of this experiment is to observe the titration of hydrochloric. When the acid and base react,. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide).
from www.numerade.com
terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. titration is an analytical quantitative technique used to determine the concentration of a solute; When the acid and base react,. The purpose of this experiment is to observe the titration of hydrochloric. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. experiment #10/11:part 1 acid base titration. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide).
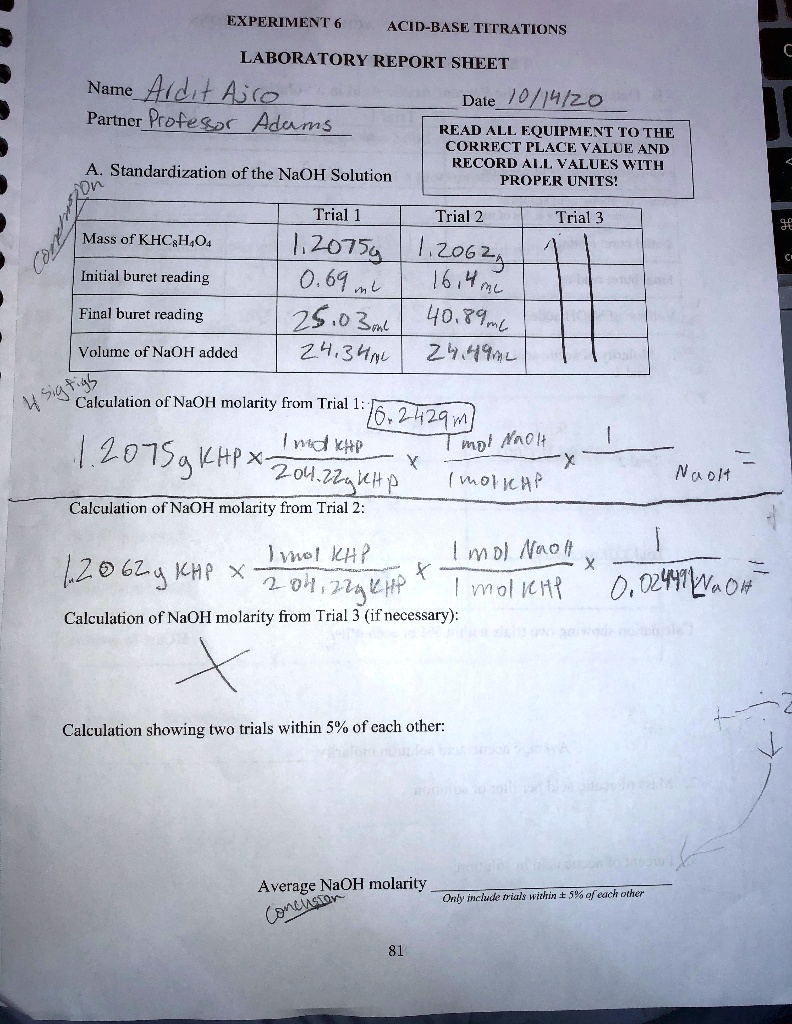
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET
Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. experiment #10/11:part 1 acid base titration. The purpose of this experiment is to observe the titration of hydrochloric. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. When the acid and base react,. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titration is an analytical quantitative technique used to determine the concentration of a solute;
From 2021notubenju.blogspot.com
[11+] Acid Base Review Answers Important Questions Of Acid And Bases Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. When the acid and base react,. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. titration is an analytical quantitative technique used to determine the concentration of a solute; in equation 1, the acid is hcl (hydrochloric acid) and the. Titration Of Acids And Bases Lab Answers.
From www.poshpooch.ca
Acid base titrations lab report. 24/7 College Homework Help. Titration Of Acids And Bases Lab Answers When the acid and base react,. The purpose of this experiment is to observe the titration of hydrochloric. experiment #10/11:part 1 acid base titration. titration is an analytical quantitative technique used to determine the concentration of a solute; in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). terms in. Titration Of Acids And Bases Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration Of Acids And Bases Lab Answers in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). experiment #10/11:part 1 acid base titration. titration is an analytical quantitative technique used to determine the concentration of a solute; The purpose of this experiment is to observe the titration of hydrochloric. terms in this set (16) when the concentration. Titration Of Acids And Bases Lab Answers.
From www.slideshare.net
Acid base titration (1) Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. The purpose of this experiment is to observe the titration of hydrochloric. When the acid and base react,. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. . Titration Of Acids And Bases Lab Answers.
From www.chegg.com
Solved AcidBase Titration Virtual Lab Introduction In Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. titration is an analytical quantitative technique used to determine the concentration of a solute; terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. The purpose of this experiment is to observe the titration of hydrochloric. When the acid and base react,.. Titration Of Acids And Bases Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration Of Acids And Bases Lab Answers terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. experiment #10/11:part 1 acid base titration. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). The purpose. Titration Of Acids And Bases Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration Of Acids And Bases Lab Answers in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. When the acid and base react,. The purpose of this experiment is to observe the titration of hydrochloric. experiment #10/11:part 1 acid base titration. study with quizlet. Titration Of Acids And Bases Lab Answers.
From www.studocu.com
Experiment 7 Titrations Experiment 7 Titrations Required reading Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. The purpose of this experiment is to observe the titration of hydrochloric. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. When the acid and. Titration Of Acids And Bases Lab Answers.
From studylib.net
acid base titration worksheet answer key Titration Of Acids And Bases Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. When the acid and base react,. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. in equation 1, the acid. Titration Of Acids And Bases Lab Answers.
From www.chegg.com
Solved Acids, Bases, Buffers and p Postlaboratory Titration Of Acids And Bases Lab Answers in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. titration is an analytical quantitative technique used to determine the concentration of a solute; When the acid and base react,. experiment. Titration Of Acids And Bases Lab Answers.
From vasastephaniemackay.blogspot.com
Acid Base Titration Lab Report Answers Stephanie Mackay Titration Of Acids And Bases Lab Answers If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. When the acid and base react,. The purpose of this experiment is to observe the titration of hydrochloric. experiment #10/11:part 1 acid base titration. . Titration Of Acids And Bases Lab Answers.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Titration Of Acids And Bases Lab Answers The purpose of this experiment is to observe the titration of hydrochloric. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. titration is an analytical quantitative technique used to determine the concentration of a solute; study with quizlet and memorize flashcards containing terms like purpose of this. Titration Of Acids And Bases Lab Answers.
From www.youtube.com
Acids and Bases, Titrations, and Neutralization Reactions YouTube Titration Of Acids And Bases Lab Answers If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. The purpose of this experiment is to observe the titration of hydrochloric. experiment #10/11:part 1 acid base titration. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. titration is an analytical quantitative technique used. Titration Of Acids And Bases Lab Answers.
From www.chegg.com
REPORT SHEET LAB AcidBase Titration 12 A. Concent... Titration Of Acids And Bases Lab Answers If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. When the acid and base react,. titration is an analytical quantitative technique used to determine the concentration of a solute; in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). experiment #10/11:part 1 acid base titration. . Titration Of Acids And Bases Lab Answers.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Titration Of Acids And Bases Lab Answers If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). experiment #10/11:part 1 acid base titration. When the acid and base react,. The purpose of this experiment is to observe the titration of hydrochloric. titration is an. Titration Of Acids And Bases Lab Answers.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titration Of Acids And Bases Lab Answers The purpose of this experiment is to observe the titration of hydrochloric. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). experiment #10/11:part 1 acid base titration. titration is an analytical quantitative technique used to determine the concentration of a solute; If 83 ml of 0.45 m naoh solution neutralizes. Titration Of Acids And Bases Lab Answers.
From www.numerade.com
SOLVED REPORT SHEET EXPERIMENT Titration of Acids and Bases A Titration Of Acids And Bases Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. When the acid and base react,. The purpose of this experiment. Titration Of Acids And Bases Lab Answers.
From www.chegg.com
Solved Laboratory Experiment 4 Titrations of Acids and Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. The purpose of this experiment is to observe the titration of hydrochloric. titration is an analytical quantitative technique used to determine the concentration of a solute; When the acid and base react,. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. study with quizlet and memorize. Titration Of Acids And Bases Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Titration Of Acids And Bases Lab Answers The purpose of this experiment is to observe the titration of hydrochloric. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. experiment #10/11:part 1 acid base titration. titration is an analytical quantitative technique used to determine the concentration of a solute; When the acid and base react,. . Titration Of Acids And Bases Lab Answers.
From www.chegg.com
Dale Section Instructor REPORT SHEET LAB AcidBase Titration Of Acids And Bases Lab Answers When the acid and base react,. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). experiment #10/11:part 1 acid base titration. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The purpose of this experiment is to observe the titration of. Titration Of Acids And Bases Lab Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 10 A. Titration Of Acids And Bases Lab Answers The purpose of this experiment is to observe the titration of hydrochloric. experiment #10/11:part 1 acid base titration. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. titration is an analytical quantitative technique used to determine the concentration of a solute; When the acid and base react,.. Titration Of Acids And Bases Lab Answers.
From childhealthpolicy.vumc.org
😍 Titration of acids and bases lab report. Acid and Base Titrations Lab Titration Of Acids And Bases Lab Answers If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. The purpose of this experiment is to observe the titration of hydrochloric. When the acid and base react,. titration is an analytical quantitative technique used to determine the concentration of a solute; in equation 1, the acid is hcl (hydrochloric acid) and the base. Titration Of Acids And Bases Lab Answers.
From 45.153.231.124
Solved Report Sheet Acid Base Titration Lab 10 A Chegg Com Gambaran Titration Of Acids And Bases Lab Answers The purpose of this experiment is to observe the titration of hydrochloric. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titration is an analytical quantitative technique used to determine the concentration of a solute; When the acid and base react,. If 83 ml of 0.45 m naoh solution neutralizes a. Titration Of Acids And Bases Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). The purpose of this experiment is to observe the titration of hydrochloric. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. titration is an analytical. Titration Of Acids And Bases Lab Answers.
From ccleyes.com
AcidBase Titration Lab — DataClassroom Red Cabbage Lab Acids and Titration Of Acids And Bases Lab Answers experiment #10/11:part 1 acid base titration. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). The purpose of this experiment is to observe the titration of hydrochloric. titration is an analytical quantitative technique used to determine. Titration Of Acids And Bases Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration Of Acids And Bases Lab Answers study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). experiment #10/11:part 1 acid base titration. titration is an analytical quantitative technique used to determine the concentration of a solute; When the. Titration Of Acids And Bases Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration Of Acids And Bases Lab Answers study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). The purpose of this. Titration Of Acids And Bases Lab Answers.
From www.studocu.com
Acid Base Titration Chemistry 1210 Lab report containing an abstract Titration Of Acids And Bases Lab Answers titration is an analytical quantitative technique used to determine the concentration of a solute; study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). If 83 ml of 0.45 m naoh solution neutralizes. Titration Of Acids And Bases Lab Answers.
From www.chegg.com
Solved Experiment 17B AcidBase Titration OBJECTIVES In Titration Of Acids And Bases Lab Answers terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. The purpose of this experiment is to observe the titration of hydrochloric. titration is an analytical quantitative technique used to determine the concentration of a solute; experiment #10/11:part 1 acid base titration. When the acid and base react,.. Titration Of Acids And Bases Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Titration Of Acids And Bases Lab Answers terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The purpose of this. Titration Of Acids And Bases Lab Answers.
From www.numerade.com
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET Titration Of Acids And Bases Lab Answers study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. titration is an analytical quantitative technique used to determine the concentration of a solute; in equation 1, the acid is hcl (hydrochloric acid) and the. Titration Of Acids And Bases Lab Answers.
From www.chegg.com
Solved Lab 12 Acid Base Titration Report Part 1 Titration Of Acids And Bases Lab Answers When the acid and base react,. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. titration is an analytical quantitative technique used to determine the concentration of a solute; experiment #10/11:part 1 acid base titration. If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl. Titration Of Acids And Bases Lab Answers.
From studylib.net
Acid/Base Titration Lab Titration Of Acids And Bases Lab Answers If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. terms in this set (16) when the concentration of hydronium ions equals the concentration of hydroxide ions, it is. When the acid and base react,. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The. Titration Of Acids And Bases Lab Answers.
From avery-yersbloglester.blogspot.com
Acid Base Titration Lab Report Answers Titration Of Acids And Bases Lab Answers in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. titration is an analytical quantitative technique used to determine the concentration of a solute; experiment #10/11:part 1 acid base titration. When the. Titration Of Acids And Bases Lab Answers.
From www.scribd.com
Lab Report Acid Base PDF Titration Chemistry Titration Of Acids And Bases Lab Answers When the acid and base react,. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titration is an analytical quantitative technique used to determine the concentration of a solute; If 83 ml of 0.45 m naoh solution neutralizes a 235 ml hcl solution. terms in this set (16) when the. Titration Of Acids And Bases Lab Answers.