Emission Spectra Gas Lamp . The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. • find the wavelength of a peak of. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. This spectrum consists of seven distinct emission lines (the first two. By comparing the emission spectrum of natural light to that produced by incandescent,. Emission spectra are not the only kind of spectra that are useful in identifying gases. • measure the emission spectrum of a source of light using the digital spectrometer. When hydrogen gas is placed. There are also absorption spectra, which are the opposite of. By the end of this experiment, you will be able to:
from byjus.com
Emission spectra are not the only kind of spectra that are useful in identifying gases. By the end of this experiment, you will be able to: The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. There are also absorption spectra, which are the opposite of. By comparing the emission spectrum of natural light to that produced by incandescent,. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. When hydrogen gas is placed. • measure the emission spectrum of a source of light using the digital spectrometer. This spectrum consists of seven distinct emission lines (the first two. • find the wavelength of a peak of.
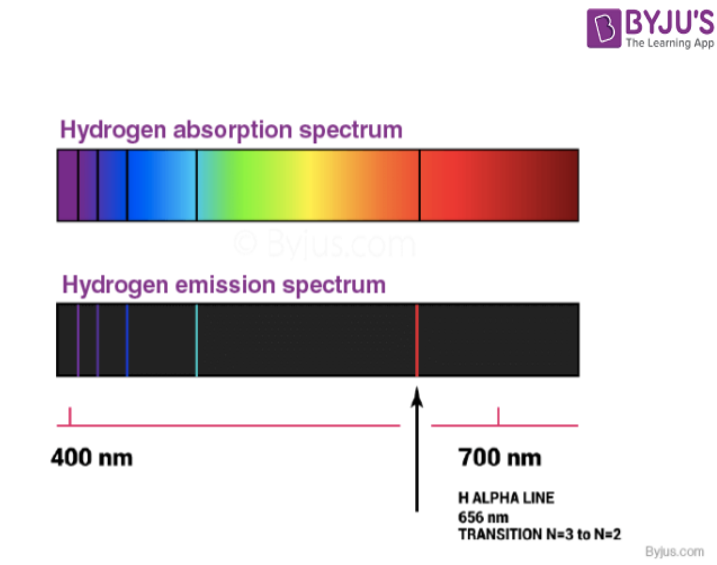
Atomic Spectra (Emission Spectrum & Absorption Spectra) Detailed
Emission Spectra Gas Lamp When hydrogen gas is placed. There are also absorption spectra, which are the opposite of. This spectrum consists of seven distinct emission lines (the first two. By the end of this experiment, you will be able to: Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. Emission spectra are not the only kind of spectra that are useful in identifying gases. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. By comparing the emission spectrum of natural light to that produced by incandescent,. • measure the emission spectrum of a source of light using the digital spectrometer. • find the wavelength of a peak of.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Gas Lamp The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. By the end of this experiment, you will be able to: • measure the emission spectrum of a source of light using the digital spectrometer. Emission spectrum from a cu hollow cathode lamp in. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectra of (a) 2, (b) 4, (c) 3 and (d) 5. Download Emission Spectra Gas Lamp There are also absorption spectra, which are the opposite of. By the end of this experiment, you will be able to: The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission spectrum from a cu hollow cathode lamp in which cu atoms are. Emission Spectra Gas Lamp.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Gas Lamp By comparing the emission spectrum of natural light to that produced by incandescent,. • measure the emission spectrum of a source of light using the digital spectrometer. This spectrum consists of seven distinct emission lines (the first two. When hydrogen gas is placed. Emission spectra are not the only kind of spectra that are useful in identifying gases. • find. Emission Spectra Gas Lamp.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Gas Lamp Emission spectra are not the only kind of spectra that are useful in identifying gases. This spectrum consists of seven distinct emission lines (the first two. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is. Emission Spectra Gas Lamp.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Gas Lamp There are also absorption spectra, which are the opposite of. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. Emission spectra are not the only kind of spectra that are useful in identifying gases. • measure the emission spectrum of a source of light using the digital spectrometer. By the end. Emission Spectra Gas Lamp.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Gas Lamp When hydrogen gas is placed. By the end of this experiment, you will be able to: This spectrum consists of seven distinct emission lines (the first two. • measure the emission spectrum of a source of light using the digital spectrometer. Emission spectra are not the only kind of spectra that are useful in identifying gases. By comparing the emission. Emission Spectra Gas Lamp.
From www.researchgate.net
UVvisible absorption spectra of HA fractions and Xenon lamp emission Emission Spectra Gas Lamp This spectrum consists of seven distinct emission lines (the first two. • measure the emission spectrum of a source of light using the digital spectrometer. By the end of this experiment, you will be able to: Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. By comparing the emission spectrum of. Emission Spectra Gas Lamp.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Gas Lamp This spectrum consists of seven distinct emission lines (the first two. • measure the emission spectrum of a source of light using the digital spectrometer. By the end of this experiment, you will be able to: • find the wavelength of a peak of. By comparing the emission spectrum of natural light to that produced by incandescent,. The emission spectrum. Emission Spectra Gas Lamp.
From www.mcphersoninc.com
Open Channel Electron Multiplier McPherson Emission Spectra Gas Lamp By the end of this experiment, you will be able to: There are also absorption spectra, which are the opposite of. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. • find the wavelength of a peak of. The emission spectrum (or line spectrum) of a chemical element is the unique. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectra of m1 and o1 Emission spectra and quantum yields a Emission Spectra Gas Lamp By the end of this experiment, you will be able to: Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. There are also absorption spectra, which are the opposite of. • measure the emission spectrum of a source of light using the digital spectrometer. When hydrogen gas is placed. This spectrum. Emission Spectra Gas Lamp.
From ar.inspiredpencil.com
Spectrum Noble Gases Emission Spectra Gas Lamp There are also absorption spectra, which are the opposite of. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. By the end of this experiment,. Emission Spectra Gas Lamp.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Gas Lamp • measure the emission spectrum of a source of light using the digital spectrometer. This spectrum consists of seven distinct emission lines (the first two. Emission spectra are not the only kind of spectra that are useful in identifying gases. There are also absorption spectra, which are the opposite of. The emission spectrum (or line spectrum) of a chemical element. Emission Spectra Gas Lamp.
From cooptata.weebly.com
Atomic emission spectrum vs continuous spectrum cooptata Emission Spectra Gas Lamp When hydrogen gas is placed. This spectrum consists of seven distinct emission lines (the first two. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. By the end of this experiment, you will be able to: By comparing the emission spectrum of natural light to that produced by incandescent,. • find. Emission Spectra Gas Lamp.
From www.researchgate.net
Spectrum of vacuumUV (183 nm) and UVC (252 nm) wavelengths emitted by Emission Spectra Gas Lamp • measure the emission spectrum of a source of light using the digital spectrometer. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. By comparing the emission spectrum of natural light to that produced by incandescent,. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Emission Spectra Gas Lamp.
From www.researchgate.net
(a) Emission spectra of the lamp, (b) XRD, (c) FTIR spectra, (d) N2 Emission Spectra Gas Lamp • measure the emission spectrum of a source of light using the digital spectrometer. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. By the end of this experiment, you will be able to: By comparing the emission spectrum of natural light to. Emission Spectra Gas Lamp.
From www.sciencephoto.com
HHeHg emission spectra Stock Image C017/7260 Science Photo Library Emission Spectra Gas Lamp When hydrogen gas is placed. This spectrum consists of seven distinct emission lines (the first two. Emission spectra are not the only kind of spectra that are useful in identifying gases. • measure the emission spectrum of a source of light using the digital spectrometer. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectrum of a mercury vapor lamp. Download Scientific Diagram Emission Spectra Gas Lamp By comparing the emission spectrum of natural light to that produced by incandescent,. Emission spectra are not the only kind of spectra that are useful in identifying gases. When hydrogen gas is placed. By the end of this experiment, you will be able to: • measure the emission spectrum of a source of light using the digital spectrometer. The emission. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectra of the lamp at acceleration voltages ranging from 1 to Emission Spectra Gas Lamp Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. By comparing the emission spectrum of natural light to that produced. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectrum of LED lamp (A) and absorption and emission spectra Emission Spectra Gas Lamp • find the wavelength of a peak of. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission spectra are not the only kind of spectra that are useful in identifying gases. There are also absorption spectra, which are the opposite of. By. Emission Spectra Gas Lamp.
From www.thoughtco.com
Balmer Series Definition in Science Emission Spectra Gas Lamp By comparing the emission spectrum of natural light to that produced by incandescent,. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. There are also absorption spectra, which are the opposite of. When hydrogen gas is placed. • measure the emission spectrum of a source of light using the digital spectrometer.. Emission Spectra Gas Lamp.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Gas Lamp Emission spectra are not the only kind of spectra that are useful in identifying gases. • find the wavelength of a peak of. • measure the emission spectrum of a source of light using the digital spectrometer. By the end of this experiment, you will be able to: By comparing the emission spectrum of natural light to that produced by. Emission Spectra Gas Lamp.
From www.youtube.com
Discharge Tubes and Emission Spectra YouTube Emission Spectra Gas Lamp This spectrum consists of seven distinct emission lines (the first two. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. • measure the emission spectrum of a source of light using the digital spectrometer. • find the wavelength of a peak of. When hydrogen gas is placed. The emission spectrum (or. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectra of gas flame and of the LED used for calibration Emission Spectra Gas Lamp Emission spectra are not the only kind of spectra that are useful in identifying gases. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. By comparing the emission spectrum of natural light to that produced by incandescent,. • measure the emission spectrum of a source of light using the digital spectrometer.. Emission Spectra Gas Lamp.
From www.researchgate.net
Optical emission spectra recorded in H2 /SiH4 miture gas glow discharge Emission Spectra Gas Lamp There are also absorption spectra, which are the opposite of. By comparing the emission spectrum of natural light to that produced by incandescent,. This spectrum consists of seven distinct emission lines (the first two. By the end of this experiment, you will be able to: When hydrogen gas is placed. • measure the emission spectrum of a source of light. Emission Spectra Gas Lamp.
From www.researchgate.net
a Emission spectrum of the lamp and absorption spectra for SMT, SMD Emission Spectra Gas Lamp This spectrum consists of seven distinct emission lines (the first two. • find the wavelength of a peak of. Emission spectra are not the only kind of spectra that are useful in identifying gases. • measure the emission spectrum of a source of light using the digital spectrometer. When hydrogen gas is placed. The emission spectrum (or line spectrum) of. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectra of UVA lamp (a) and VIS lamp without (1) and with (2 Emission Spectra Gas Lamp • find the wavelength of a peak of. This spectrum consists of seven distinct emission lines (the first two. Emission spectra are not the only kind of spectra that are useful in identifying gases. When hydrogen gas is placed. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. By the end. Emission Spectra Gas Lamp.
From www.researchgate.net
Eu lamp (black), Nd lamp (red), and Gd lamp (blue) emission spectra Emission Spectra Gas Lamp Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. There are also absorption spectra, which are the opposite of. This spectrum consists of seven distinct emission lines (the first two. • measure the emission spectrum of a source of light using the digital spectrometer. When hydrogen gas is placed. By the. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectra of the discharge when helium flows (purified) or does Emission Spectra Gas Lamp By comparing the emission spectrum of natural light to that produced by incandescent,. • find the wavelength of a peak of. There are also absorption spectra, which are the opposite of. • measure the emission spectrum of a source of light using the digital spectrometer. By the end of this experiment, you will be able to: Emission spectrum from a. Emission Spectra Gas Lamp.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Emission Spectra Gas Lamp Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. • find the wavelength of a peak of. There are also absorption spectra, which are the opposite of. This spectrum consists of seven distinct emission lines (the first two. Emission spectra are not the only kind of spectra that are useful in. Emission Spectra Gas Lamp.
From www.nagwa.com
Question Video Identifying the Emission Spectrum Corresponding to an Emission Spectra Gas Lamp By the end of this experiment, you will be able to: There are also absorption spectra, which are the opposite of. • measure the emission spectrum of a source of light using the digital spectrometer. By comparing the emission spectrum of natural light to that produced by incandescent,. This spectrum consists of seven distinct emission lines (the first two. •. Emission Spectra Gas Lamp.
From byjus.com
Atomic Spectra (Emission Spectrum & Absorption Spectra) Detailed Emission Spectra Gas Lamp The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. By the end of this experiment, you will be able to: • measure the emission spectrum of a source of light using the digital spectrometer. This spectrum consists of. Emission Spectra Gas Lamp.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra Gas Lamp By comparing the emission spectrum of natural light to that produced by incandescent,. Emission spectra are not the only kind of spectra that are useful in identifying gases. • find the wavelength of a peak of. This spectrum consists of seven distinct emission lines (the first two. When hydrogen gas is placed. There are also absorption spectra, which are the. Emission Spectra Gas Lamp.
From www.researchgate.net
Emission spectra of the P1 solution (1 mg/mL) at different excitation Emission Spectra Gas Lamp The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. This spectrum consists of seven distinct emission lines (the first two. There are also absorption spectra,. Emission Spectra Gas Lamp.
From www.chegg.com
Atomic Emission Spectral Data for Selected Gases Emission Spectra Gas Lamp Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. • measure the emission spectrum of a source of light using the digital spectrometer. Emission spectra are not the only kind of spectra that are useful in identifying gases. By the end of this experiment, you will be able to: By comparing. Emission Spectra Gas Lamp.
From www.alamy.com
emission spectrum of neon Stock Photo 131728 Alamy Emission Spectra Gas Lamp • find the wavelength of a peak of. There are also absorption spectra, which are the opposite of. When hydrogen gas is placed. Emission spectrum from a cu hollow cathode lamp in which cu atoms are present in the gas phase. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Emission Spectra Gas Lamp.