Graphite Boiling Point . learn how diamond and graphite are different forms of carbon with different properties and melting points. Its melting point is 3600 °c, and it has low density, strength, and hardness. Learn about its properties, such as high melting point,. Diamond has a high melting point. graphite is also used in a fibrous form for various plastics. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. Carbon has very high melting and boiling points. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. graphite has a giant covalent structure with weak forces between the layers.
from general.chemistrysteps.com
Carbon has very high melting and boiling points. learn how diamond and graphite are different forms of carbon with different properties and melting points. Learn about its properties, such as high melting point,. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. graphite is also used in a fibrous form for various plastics. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. Its melting point is 3600 °c, and it has low density, strength, and hardness. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. Diamond has a high melting point. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such.
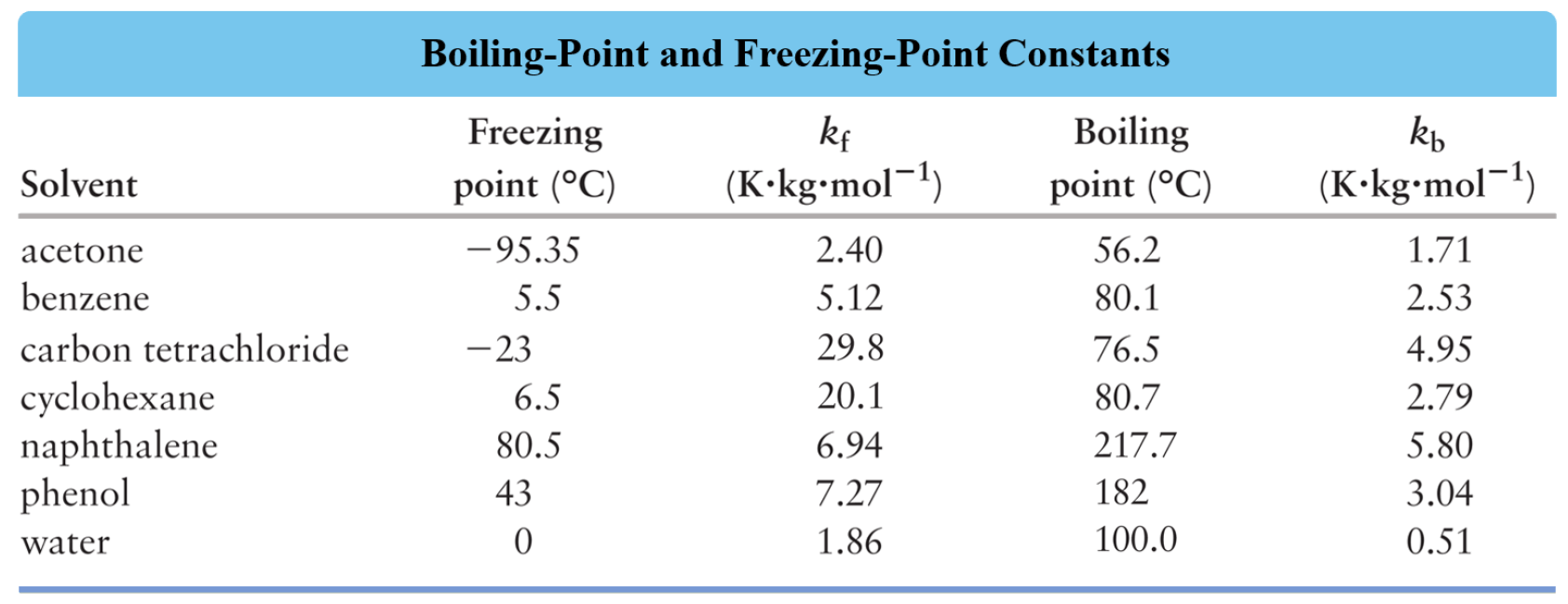
Boiling Point Elevation Chemistry Steps
Graphite Boiling Point Carbon has very high melting and boiling points. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. Diamond has a high melting point. graphite is also used in a fibrous form for various plastics. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. Learn about its properties, such as high melting point,. learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. graphite has a giant covalent structure with weak forces between the layers. Its melting point is 3600 °c, and it has low density, strength, and hardness. Carbon has very high melting and boiling points.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Graphite Boiling Point graphite is a form of carbon with a layered structure of hexagonal rings of atoms. Diamond has a high melting point. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. graphite has a giant covalent structure with weak forces between the layers. graphite is also used in a. Graphite Boiling Point.
From chem.libretexts.org
Fundamentals of Phase Transitions Chemistry LibreTexts Graphite Boiling Point graphite is also used in a fibrous form for various plastics. learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite has a giant covalent structure with weak forces between the layers. Carbon has very high melting and boiling points. Learn about its properties, such as high melting point,. . Graphite Boiling Point.
From www.youtube.com
ChemII Lecture9 Melting and boiling points YouTube Graphite Boiling Point Carbon has very high melting and boiling points. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. learn how diamond and graphite are different forms of carbon with different properties and melting points. Diamond has. Graphite Boiling Point.
From socratic.org
What is the relation between critical temperature and boiling point or vapor pressure? Socratic Graphite Boiling Point Its melting point is 3600 °c, and it has low density, strength, and hardness. Diamond has a high melting point. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. Learn about its properties, such as high melting point,. graphite is a form of carbon with a layered structure of. Graphite Boiling Point.
From wou.edu
CH105 Chapter 9 Organic Compounds of Oxygen Chemistry Graphite Boiling Point graphite is a form of carbon with a layered structure of hexagonal rings of atoms. graphite is also used in a fibrous form for various plastics. Learn about its properties, such as high melting point,. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. graphite has a giant. Graphite Boiling Point.
From www.jove.com
11369.jpg Graphite Boiling Point Learn about its properties, such as high melting point,. Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. graphite is also used in a fibrous form for various plastics. graphite is a form of carbon with a. Graphite Boiling Point.
From www.saatchiart.com
BOILING GRAPHITE/ GRAPHITE EN ÉBULLITION Painting by Claude Lessard Saatchi Art Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. Its melting point is 3600 °c, and it has low density, strength, and hardness. Carbon has very high melting and boiling points. Learn about its properties, such as high melting point,. learn how the covalent bonding and layer structure of graphite and. Graphite Boiling Point.
From material-properties.org
Graphite Density, Strength, Hardness, Melting Point Graphite Boiling Point graphite is a form of carbon with a layered structure of hexagonal rings of atoms. Diamond has a high melting point. Carbon has very high melting and boiling points. Learn about its properties, such as high melting point,. graphite has a giant covalent structure with weak forces between the layers. learn how diamond and graphite are different. Graphite Boiling Point.
From www.engineeringtoolbox.com
Hydrocarbons, alcohols and acids boiling points Graphite Boiling Point learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. Carbon has very high melting and boiling points. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. Its melting point. Graphite Boiling Point.
From wisc.pb.unizin.org
Day 11 Molecular Structure Isomers Chemistry 109, Fall 2020 Graphite Boiling Point Carbon has very high melting and boiling points. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. Diamond has a high melting point. graphite is also used in a fibrous form for various plastics. graphite is a form of carbon with a layered structure of hexagonal rings of. Graphite Boiling Point.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points Master Organic Chemistry Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. Learn about its properties, such as high melting point,. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial. Graphite Boiling Point.
From www.slideshare.net
Chem matters ch7_covalent_bonding Graphite Boiling Point Diamond has a high melting point. Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. learn about the structure, density,. Graphite Boiling Point.
From www.nuclear-power.com
Carbon Melting Point Boiling Point Graphite Boiling Point learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. Carbon has very high melting and boiling points. Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is a. Graphite Boiling Point.
From www.passmyexams.co.uk
Physical Properties of Alkanes Easy exam revision notes for GSCE Chemistry Graphite Boiling Point Learn about its properties, such as high melting point,. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is a. Graphite Boiling Point.
From chem2u.blogspot.com
chem2U Individual Assignment Alkanes Graphite Boiling Point Learn about its properties, such as high melting point,. Diamond has a high melting point. Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. graphite is a form of carbon with a layered structure of hexagonal rings of atoms.. Graphite Boiling Point.
From brainly.com
This graph shows the melting and boiling points of the alkali metals. Think about Francium, do Graphite Boiling Point Diamond has a high melting point. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite has a giant covalent. Graphite Boiling Point.
From www.slideserve.com
PPT Melting Point and Boiling Point PowerPoint Presentation, free download ID2326555 Graphite Boiling Point learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. learn how diamond and graphite are different forms of carbon. Graphite Boiling Point.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite has a giant covalent structure with weak forces between the layers. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. learn how the covalent bonding and layer structure of graphite and diamond affect. Graphite Boiling Point.
From chemistry.stackexchange.com
phase What is the melting point of diamond? Chemistry Stack Exchange Graphite Boiling Point Its melting point is 3600 °c, and it has low density, strength, and hardness. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. graphite is also used in a fibrous form for various plastics. learn how diamond and graphite are different forms of carbon with different properties and. Graphite Boiling Point.
From www.wou.edu
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite has a giant covalent structure with weak forces between the layers. Diamond has a high melting point. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. Carbon has very high melting and. Graphite Boiling Point.
From material-properties.org
Carbon Thermal Properties Melting Point Thermal Conductivity Expansion Graphite Boiling Point graphite is also used in a fibrous form for various plastics. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. Learn about its properties, such as high melting point,. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. Carbon has very high melting. Graphite Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Graphite Boiling Point learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. graphite is also used in a fibrous form for various plastics. learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite is a form of carbon with a layered structure of hexagonal. Graphite Boiling Point.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart Graphite Boiling Point graphite is a form of carbon with a layered structure of hexagonal rings of atoms. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. Learn about its properties, such as high melting point,. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. . Graphite Boiling Point.
From www.chemix-chemistry-software.com
Melting point and boiling point of elements Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. Diamond has a high melting point. Its melting point is 3600 °c,. Graphite Boiling Point.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols Graphite Boiling Point graphite is also used in a fibrous form for various plastics. learn how diamond and graphite are different forms of carbon with different properties and melting points. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. learn how the covalent bonding and layer structure of graphite and. Graphite Boiling Point.
From slideplayer.com
Melting and boiling point giant structures ppt download Graphite Boiling Point graphite is a form of carbon with a hexagonal structure and high thermal conductivity. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite has a giant covalent structure with weak. Graphite Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Graphite Boiling Point graphite has a giant covalent structure with weak forces between the layers. graphite is a form of carbon with a layered structure of hexagonal rings of atoms. Learn about its properties, such as high melting point,. Diamond has a high melting point. Its melting point is 3600 °c, and it has low density, strength, and hardness. Carbon has. Graphite Boiling Point.
From www.slideserve.com
PPT Carbon Compounds An Introduction to Organic Chemistry PowerPoint Presentation ID9707655 Graphite Boiling Point Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. Diamond has a high melting point. Carbon has very high melting and boiling points. Learn about its properties, such as high melting point,. graphite is also used in a fibrous. Graphite Boiling Point.
From www.engineeringtoolbox.com
Boiling points of hydrocarbons, alcohols and acids Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. Diamond has a high melting point. Carbon has very high melting and boiling points. Its melting point is 3600 °c, and it has low density, strength, and hardness. graphite is also used in a fibrous form for various plastics. graphite has. Graphite Boiling Point.
From wisc.pb.unizin.org
M10Q2 Melting and Boiling Point Comparisons Chem 103/104 Resource Book Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite is also used in a fibrous form for various plastics. Learn about its properties, such as high melting point,. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. Carbon has very high melting and boiling. Graphite Boiling Point.
From chemistryjee.blogspot.com
chemistry world Alkanes Physical Properties Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite is a form of carbon with a hexagonal structure and high thermal conductivity. graphite is also used in a fibrous form for various plastics. Learn about its properties, such as high melting point,. Its melting point is 3600 °c, and. Graphite Boiling Point.
From wisc.pb.unizin.org
Melting and Boiling Point Comparisons (M10Q2) UWMadison Chemistry 103/104 Resource Book Graphite Boiling Point Diamond has a high melting point. learn how the covalent bonding and layer structure of graphite and diamond affect their physical properties, such. graphite is also used in a fibrous form for various plastics. learn about the structure, density, and other properties of graphite, a form of carbon with many industrial applications. graphite has a giant. Graphite Boiling Point.
From themachine.science
The Boiling Point of Graphite A Comprehensive Exploration Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. Diamond has a high melting point. graphite has a giant covalent structure with weak forces between the layers. Carbon has very high melting and boiling points. graphite is also used in a fibrous form for various plastics. graphite is a. Graphite Boiling Point.
From www.studypool.com
SOLUTION Difference Between Diamond And Graphite Studypool Graphite Boiling Point learn how diamond and graphite are different forms of carbon with different properties and melting points. graphite is also used in a fibrous form for various plastics. Learn about its properties, such as high melting point,. Carbon has very high melting and boiling points. Diamond has a high melting point. graphite is a form of carbon with. Graphite Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Graphite Boiling Point Learn about its properties, such as high melting point,. graphite has a giant covalent structure with weak forces between the layers. graphite is also used in a fibrous form for various plastics. Its melting point is 3600 °c, and it has low density, strength, and hardness. learn about the structure, density, and other properties of graphite, a. Graphite Boiling Point.