Emission Spectrum Formula . The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. This process is called atomic. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Substituting the relationship between the frequency, wavelength, and the speed of. Planck's equation states that the energy of a photon is proportional to its frequency. The characteristic spectrum of each element can be used in. The actual wavelengths of the lines are predictably. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the.
from www.youtube.com
Planck's equation states that the energy of a photon is proportional to its frequency. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. The figure below shows the. The actual wavelengths of the lines are predictably. This process is called atomic. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Substituting the relationship between the frequency, wavelength, and the speed of. The characteristic spectrum of each element can be used in. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the.
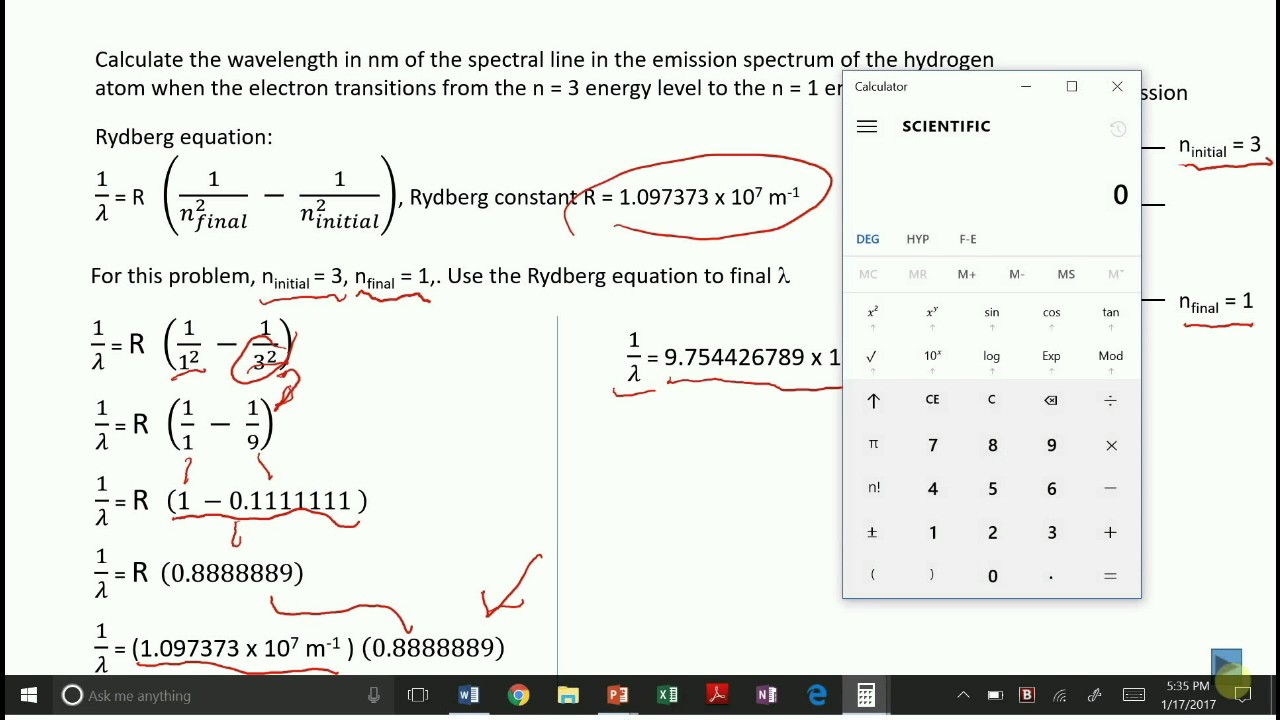
The emission spectrum of the hydrogen atom and the Rydberg equation
Emission Spectrum Formula An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. Substituting the relationship between the frequency, wavelength, and the speed of. The actual wavelengths of the lines are predictably. The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The figure below shows the. This process is called atomic. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The characteristic spectrum of each element can be used in. Planck's equation states that the energy of a photon is proportional to its frequency. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains.
From socratic.org
How did Bohr theory explain the emission spectrum of hydrogen? Socratic Emission Spectrum Formula Planck's equation states that the energy of a photon is proportional to its frequency. Substituting the relationship between the frequency, wavelength, and the speed of. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The amount of light absorbed is proportional to the concentration of the element in the. Emission Spectrum Formula.
From www.youtube.com
Emission spectra continuous vs line spectrum and the Rydberg formula Emission Spectrum Formula The figure below shows the. Substituting the relationship between the frequency, wavelength, and the speed of. This process is called atomic. The characteristic spectrum of each element can be used in. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. Emission. Emission Spectrum Formula.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID Emission Spectrum Formula The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. Substituting the relationship between the frequency, wavelength, and the speed of. This process is called atomic. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different. Emission Spectrum Formula.
From www.youtube.com
Emission spectrum of hydrogen Physical Processes MCAT Khan Emission Spectrum Formula Substituting the relationship between the frequency, wavelength, and the speed of. The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. The actual wavelengths of the lines are predictably. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. An. Emission Spectrum Formula.
From classnotes.org.in
Absorption and Emission Spectra Chemistry, Class 11, Structure of Atom Emission Spectrum Formula Planck's equation states that the energy of a photon is proportional to its frequency. This process is called atomic. Substituting the relationship between the frequency, wavelength, and the speed of. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. An emission spectrum of an element is the unique pattern. Emission Spectrum Formula.
From discover.hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectrum Formula An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy, which uses. Emission Spectrum Formula.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectrum Formula The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. The characteristic spectrum of each element can be used in. The figure below shows the. Substituting the relationship between the frequency, wavelength, and the speed of. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide. Emission Spectrum Formula.
From chem.libretexts.org
5.2 The Spectrum Chemistry LibreTexts Emission Spectrum Formula The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. The characteristic spectrum of each element can be used in. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An emission spectrum of an element is the unique pattern of light obtained when the element. Emission Spectrum Formula.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission Emission Spectrum Formula The actual wavelengths of the lines are predictably. The characteristic spectrum of each element can be used in. This process is called atomic. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The figure below shows the. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation. Emission Spectrum Formula.
From jpl.nasa.gov
Educator Guide Math of the Expanding Universe NASA/JPL Edu Emission Spectrum Formula Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Planck's equation states that the energy of a photon is proportional to its frequency. The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. Substituting the relationship between the frequency,. Emission Spectrum Formula.
From www.researchgate.net
The emission spectrum of the 385 nm UVA1 source (dotted line) and the Emission Spectrum Formula The spectroscope separates the light of varying wavelengths and we observe a line spectrum. This process is called atomic. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The actual wavelengths of the lines are predictably. An atomic emission spectrum is the pattern of lines formed when. Emission Spectrum Formula.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectrum Formula Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. The characteristic spectrum of each element can be used in. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different. Emission Spectrum Formula.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectrum Formula The spectroscope separates the light of varying wavelengths and we observe a line spectrum. This process is called atomic. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. Planck's equation states that the energy of a photon is proportional to its. Emission Spectrum Formula.
From www.sciencefacts.net
Atomic Emission and Absorption Spectra Definition and Formula Emission Spectrum Formula The figure below shows the. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. An emission spectrum of an element is. Emission Spectrum Formula.
From www.pinterest.com
Absorption and emission lines Astrophysics, Space and astronomy Emission Spectrum Formula The characteristic spectrum of each element can be used in. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Planck's. Emission Spectrum Formula.
From www.youtube.com
Number of Spectral Lines Emission and Absorption Spectra YouTube Emission Spectrum Formula This process is called atomic. The figure below shows the. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The characteristic spectrum of each element can be used in. Substituting the relationship between the frequency, wavelength, and the speed of. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number. Emission Spectrum Formula.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectrum Formula Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. Planck's equation states that the energy of a photon is proportional to its frequency. This process is called atomic. An atomic emission spectrum is the pattern of lines formed when light passes. Emission Spectrum Formula.
From edurev.in
Using the Rydberg formula,calculate the wavelengths of first four Emission Spectrum Formula Substituting the relationship between the frequency, wavelength, and the speed of. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. This process is called atomic. The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. The actual wavelengths of the lines are predictably. The figure. Emission Spectrum Formula.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectrum Formula An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. The amount of. Emission Spectrum Formula.
From www.youtube.com
Calculating Wavelengths of Emitted Light in the Hydrogen Spectrum YouTube Emission Spectrum Formula The characteristic spectrum of each element can be used in. This process is called atomic. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. An emission spectrum. Emission Spectrum Formula.
From www.slideserve.com
PPT Chapter 3 Spectral lines in stars PowerPoint Presentation, free Emission Spectrum Formula The figure below shows the. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Planck's equation states that the energy of a photon is proportional to its frequency. An atomic emission spectrum is the pattern. Emission Spectrum Formula.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube Emission Spectrum Formula An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. Planck's equation states that. Emission Spectrum Formula.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Emission Spectrum Formula An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Substituting the relationship between the frequency, wavelength, and the speed of. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The amount. Emission Spectrum Formula.
From byjus.com
Atomic Spectra (Emission Spectrum & Absorption Spectra) Detailed Emission Spectrum Formula The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. This process is called atomic. The. Emission Spectrum Formula.
From www.slideserve.com
PPT Chapter 41 PowerPoint Presentation, free download ID8080 Emission Spectrum Formula An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. The characteristic spectrum of each element can be used in. An. Emission Spectrum Formula.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectrum Formula Planck's equation states that the energy of a photon is proportional to its frequency. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The characteristic spectrum of each element can be used in. This process is called atomic. The figure below shows the. Substituting the relationship between the frequency, wavelength, and the speed of. An. Emission Spectrum Formula.
From www.youtube.com
Atomic spectra , Emission and absorption spectra Hydrogen spectra and Emission Spectrum Formula The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Substituting the relationship between. Emission Spectrum Formula.
From www.researchgate.net
Emission spectral intensity and relative luminance of RPFs Download Emission Spectrum Formula The actual wavelengths of the lines are predictably. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about. Emission Spectrum Formula.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectrum Formula Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The amount of light absorbed. Emission Spectrum Formula.
From www.researchgate.net
a emission spectra; b normalized emission spectra; c UV spectrum (left Emission Spectrum Formula The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. Emission Spectrum Formula.
From www.thoughtco.com
What Is the Rydberg Equation and How Does It Work? Emission Spectrum Formula The amount of light absorbed is proportional to the concentration of the element in the sample, allowing for quantitative analysis. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The actual wavelengths of the lines are predictably. Planck's equation states that the energy of a photon is proportional to. Emission Spectrum Formula.
From www.youtube.com
The emission spectrum of the hydrogen atom and the Rydberg equation Emission Spectrum Formula Substituting the relationship between the frequency, wavelength, and the speed of. Planck's equation states that the energy of a photon is proportional to its frequency. The actual wavelengths of the lines are predictably. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron. Emission Spectrum Formula.
From www.youtube.com
Absorption and Emission Spectra of Hydrogen YouTube Emission Spectrum Formula An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Johannes rydberg, a swedish spectroscopist, derived a general formula for the. Emission Spectrum Formula.
From socratic.org
What is line emission spectrum? + Example Emission Spectrum Formula Substituting the relationship between the frequency, wavelength, and the speed of. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number. Emission Spectrum Formula.
From byjus.com
Hydrogen Spectrum Balmer SeriesDefinitionDiagram Chemistry Byju’s Emission Spectrum Formula Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Johannes rydberg, a swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from. An emission spectrum of an element is the unique pattern of light. Emission Spectrum Formula.