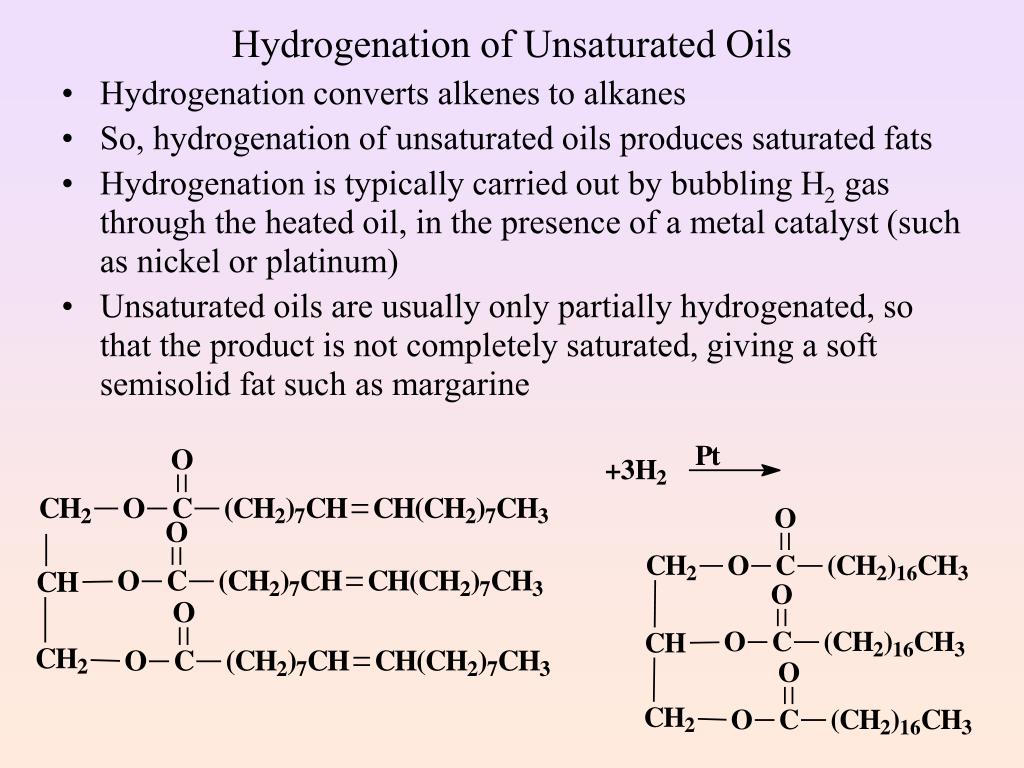
Hydrogenation Of Vegetable Oil Equation . The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Hydrogenation means adding hydrogen to a substance. This simply means that there are several double bonds present. Vegetable oils are commonly referred to as polyunsaturated. Hydrogenation and transesterification of vegetable oils with gc analysis. Liquid vegetable oils that are unsaturated will react with hydrogen at about. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. A simple hydrogenation reaction is: Recall that the addition of hydrogen to an alkene (unsaturated) results. During a hydrogenation reaction, the number of carbon. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds.
from www.slideserve.com
A simple hydrogenation reaction is: Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. Hydrogenation means adding hydrogen to a substance. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Liquid vegetable oils that are unsaturated will react with hydrogen at about. During a hydrogenation reaction, the number of carbon. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Recall that the addition of hydrogen to an alkene (unsaturated) results. Hydrogenation and transesterification of vegetable oils with gc analysis.
PPT Lipids PowerPoint Presentation, free download ID6534192
Hydrogenation Of Vegetable Oil Equation Vegetable oils are commonly referred to as polyunsaturated. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. During a hydrogenation reaction, the number of carbon. This simply means that there are several double bonds present. Hydrogenation means adding hydrogen to a substance. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. Vegetable oils are commonly referred to as polyunsaturated. A simple hydrogenation reaction is: Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Liquid vegetable oils that are unsaturated will react with hydrogen at about. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Recall that the addition of hydrogen to an alkene (unsaturated) results. Hydrogenation and transesterification of vegetable oils with gc analysis.
From studylib.net
Transfer Hydrogenation of Olive Oil Hydrogenation Of Vegetable Oil Equation Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. This simply means that there are several double bonds present. Hydrogenation and transesterification of vegetable oils with gc analysis. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. Hydrogenation is the reaction which involves the addition of one or more. Hydrogenation Of Vegetable Oil Equation.
From www.teachoo.com
[Carbon Class 10] Hydrogenation of oils Definition, Reaction Hydrogenation Of Vegetable Oil Equation This simply means that there are several double bonds present. Liquid vegetable oils that are unsaturated will react with hydrogen at about. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. The vegetable oils are unsaturated compounds containing double bonds.they are in. Hydrogenation Of Vegetable Oil Equation.
From www.researchgate.net
Flash point and autoignition temperature of several common vegetable Hydrogenation Of Vegetable Oil Equation Hydrogenation and transesterification of vegetable oils with gc analysis. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. This simply means that there are several double bonds present. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Hydrogenation. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
Hardening Vegetable Oils (Margarine) through Hydrogenation Chemistry Hydrogenation Of Vegetable Oil Equation Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. Recall that the addition of hydrogen to an alkene (unsaturated) results. This simply means that there are several double bonds present. Hydrogenation means adding hydrogen to a substance. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical. Hydrogenation Of Vegetable Oil Equation.
From www.scribd.com
Hydrogenation of Vegetable Oil To Margarine PDF Hydrogenation Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. A simple hydrogenation reaction is: The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Recall that the addition of hydrogen to an alkene (unsaturated) results. The. Hydrogenation Of Vegetable Oil Equation.
From www.teachoo.com
[Carbon Class 10] Hydrogenation of oils Definition, Reaction Hydrogenation Of Vegetable Oil Equation Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Liquid vegetable oils that are unsaturated will react with hydrogen at about. Vegetable oils are commonly referred to as polyunsaturated. This simply means that there are several double bonds present. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. The. Hydrogenation Of Vegetable Oil Equation.
From www.slideshare.net
Hydrogenation of Edible Oils Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. A simple hydrogenation reaction is: Recall that the addition of hydrogen to an alkene (unsaturated) results. Hydrogenation and transesterification of vegetable oils with gc analysis. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. During a hydrogenation reaction, the number of carbon.. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
Hydrogenation of vegetable ghee at \( 25^{\circ} \mathrm{C} \) redu Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. During a hydrogenation reaction, the number of carbon. Vegetable oils are commonly referred to as polyunsaturated. Hydrogenation and transesterification of vegetable oils with gc analysis. Recall that the addition of hydrogen to an alkene (unsaturated) results. Vegetable oils may be converted from liquids to. Hydrogenation Of Vegetable Oil Equation.
From www.researchgate.net
(PDF) Hydrogenation of Vegetable Oils Using Mixtures of Supercritical Hydrogenation Of Vegetable Oil Equation The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Hydrogenation and transesterification of vegetable oils with gc analysis. A simple hydrogenation reaction is: Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. The. Hydrogenation Of Vegetable Oil Equation.
From www.slideserve.com
PPT Chapter 13 Alkanes, Alkynes, and Aromatic Compounds PowerPoint Hydrogenation Of Vegetable Oil Equation Liquid vegetable oils that are unsaturated will react with hydrogen at about. Hydrogenation means adding hydrogen to a substance. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. During a hydrogenation reaction, the number of carbon. A simple hydrogenation reaction is: The vegetable oils are unsaturated compounds containing double bonds.they are in. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
Changing of vegetable oil into ghee HydrogenationVanaspati GheeB Hydrogenation Of Vegetable Oil Equation Hydrogenation means adding hydrogen to a substance. A simple hydrogenation reaction is: The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Vegetable oils are commonly referred to as polyunsaturated. During a hydrogenation reaction, the number of carbon. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple. Hydrogenation Of Vegetable Oil Equation.
From www.slideserve.com
PPT Partial Hydrogenation of Vegetable Oil using Membrane Reactor Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Hydrogenation. Hydrogenation Of Vegetable Oil Equation.
From www.slideserve.com
PPT Lipids PowerPoint Presentation, free download ID6534192 Hydrogenation Of Vegetable Oil Equation Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. Liquid vegetable oils that are unsaturated will react with hydrogen at about. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it. Hydrogenation Of Vegetable Oil Equation.
From studylib.net
Transfer Hydrogenation of Olive Oil Hydrogenation Of Vegetable Oil Equation Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Hydrogenation and transesterification of vegetable oils with gc analysis. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. Partial and full hydrogenation of vegetable. Hydrogenation Of Vegetable Oil Equation.
From www.scribd.com
Hydrogenation of Vegetable Oil PDF Hydrogenation Vegetable Oil Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. A simple hydrogenation reaction is: Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Hydrogenation and transesterification of vegetable oils with gc analysis. The. Hydrogenation Of Vegetable Oil Equation.
From courses.lumenlearning.com
Hydrogenation Introduction to Chemistry Hydrogenation Of Vegetable Oil Equation Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Hydrogenation and transesterification of vegetable oils with gc analysis. A simple hydrogenation reaction is: Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Recall. Hydrogenation Of Vegetable Oil Equation.
From www.researchgate.net
Composition of hydrogenated vegetable oils and vegetable oil Hydrogenation Of Vegetable Oil Equation The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. Liquid vegetable oils that are unsaturated will react with hydrogen at about. The vegetable oils are unsaturated. Hydrogenation Of Vegetable Oil Equation.
From www.doubtnut.com
A molecule of a vegetable oil (X) contains one C=C double bond. How Hydrogenation Of Vegetable Oil Equation During a hydrogenation reaction, the number of carbon. Hydrogenation and transesterification of vegetable oils with gc analysis. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Hydrogenation means adding hydrogen to a substance. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. This simply means that there are several. Hydrogenation Of Vegetable Oil Equation.
From www.researchgate.net
Structure of hydrogenated castor oil produced from hydrogenation of Hydrogenation Of Vegetable Oil Equation Vegetable oils are commonly referred to as polyunsaturated. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. The vegetable oils are unsaturated compounds containing double. Hydrogenation Of Vegetable Oil Equation.
From www.dyseg.com
Hydrogenated Vegetable Oil Chemical Formula Best Vegetable In The World Hydrogenation Of Vegetable Oil Equation Vegetable oils are commonly referred to as polyunsaturated. Hydrogenation means adding hydrogen to a substance. During a hydrogenation reaction, the number of carbon. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be. Hydrogenation Of Vegetable Oil Equation.
From byjus.com
72.Hydrogenation of vegetable oilis what kind of reaction and what Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. Hydrogenation means adding hydrogen to a substance. During a hydrogenation reaction, the number of carbon. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. The desired product might be the oil with three oleic acid substituents. Hydrogenation Of Vegetable Oil Equation.
From www.slideserve.com
PPT Alkenes PowerPoint Presentation, free download ID171306 Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. This simply means that there are. Hydrogenation Of Vegetable Oil Equation.
From www.slideserve.com
PPT Chemistry 2100 PowerPoint Presentation, free download ID6574979 Hydrogenation Of Vegetable Oil Equation Liquid vegetable oils that are unsaturated will react with hydrogen at about. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. During a hydrogenation reaction, the number of carbon. Hydrogenation and transesterification of vegetable oils with gc analysis. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it. Hydrogenation Of Vegetable Oil Equation.
From www.researchgate.net
(PDF) Electrocatalytic Hydrogenation of Vegetable Oil Hydrogenation Of Vegetable Oil Equation \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Hydrogenation and transesterification of vegetable oils with gc analysis. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Vegetable oils are commonly referred to as polyunsaturated. Liquid vegetable oils that are. Hydrogenation Of Vegetable Oil Equation.
From www.slideshare.net
Hydrogenation Hydrogenation Of Vegetable Oil Equation Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. This simply means that there are several double bonds present. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Hydrogenation. Hydrogenation Of Vegetable Oil Equation.
From www.visionlearning.com
Lipids Biology Visionlearning Hydrogenation Of Vegetable Oil Equation During a hydrogenation reaction, the number of carbon. Recall that the addition of hydrogen to an alkene (unsaturated) results. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. Hydrogenation means adding hydrogen to a substance. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. This. Hydrogenation Of Vegetable Oil Equation.
From www.slideshare.net
Hydrogenation of Edible Oils Hydrogenation Of Vegetable Oil Equation \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Vegetable oils are commonly referred to as polyunsaturated. During a hydrogenation reaction, the number of carbon. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries.. Hydrogenation Of Vegetable Oil Equation.
From www.slideshare.net
Hydrogenation of Edible Oils Hydrogenation Of Vegetable Oil Equation Hydrogenation means adding hydrogen to a substance. Hydrogenation and transesterification of vegetable oils with gc analysis. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Vegetable. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
Hydrogenation of oils to form margarine YouTube Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. Vegetable oils are commonly referred to as polyunsaturated. A simple hydrogenation reaction is: \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. This simply means that there are several double bonds present. Hydrogenation and transesterification of vegetable oils with gc analysis. Hydrogenation. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
How To Harden Vegetable Oils Through Hydrogenation Organic Chemistry Hydrogenation Of Vegetable Oil Equation This simply means that there are several double bonds present. Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. The desired product might be the oil with three oleic acid substituents (we'll abbreviate it gooo, which also might be a good description of it) so the. Vegetable oils are commonly referred to as. Hydrogenation Of Vegetable Oil Equation.
From brainly.in
Write chemical equation to represent Hydrogenation of Vegetable oils Hydrogenation Of Vegetable Oil Equation Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. Hydrogenation and transesterification of vegetable oils with gc analysis. Partial and. Hydrogenation Of Vegetable Oil Equation.
From ar.inspiredpencil.com
Hydrogenated Oil Structure Hydrogenation Of Vegetable Oil Equation Recall that the addition of hydrogen to an alkene (unsaturated) results. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. The desired product might be the. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
What is the hydrogenation of vegetable oils? 8 HYDROGEN CHEMISTRY Hydrogenation Of Vegetable Oil Equation \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. A simple hydrogenation reaction is: Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature. Liquid vegetable oils that are unsaturated will react with hydrogen at about.. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
B.10 Hydrogenation of oils (HL) YouTube Hydrogenation Of Vegetable Oil Equation A simple hydrogenation reaction is: Hydrogenation and transesterification of vegetable oils with gc analysis. Liquid vegetable oils that are unsaturated will react with hydrogen at about. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Hydrogenation is the reaction which involves the addition of one or more hydrogen atoms to double/triple bonds. Vegetable oils may be converted from. Hydrogenation Of Vegetable Oil Equation.
From www.youtube.com
HYDROGENATION OF VEGETABLE OIL YouTube Hydrogenation Of Vegetable Oil Equation Partial and full hydrogenation of vegetable oils are extremely important for the food and chemical industries. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Hydrogenation means adding hydrogen to a substance. Vegetable oils are commonly referred to as polyunsaturated. The vegetable oils are unsaturated compounds containing double bonds.they are in liquid state at room temperature.. Hydrogenation Of Vegetable Oil Equation.