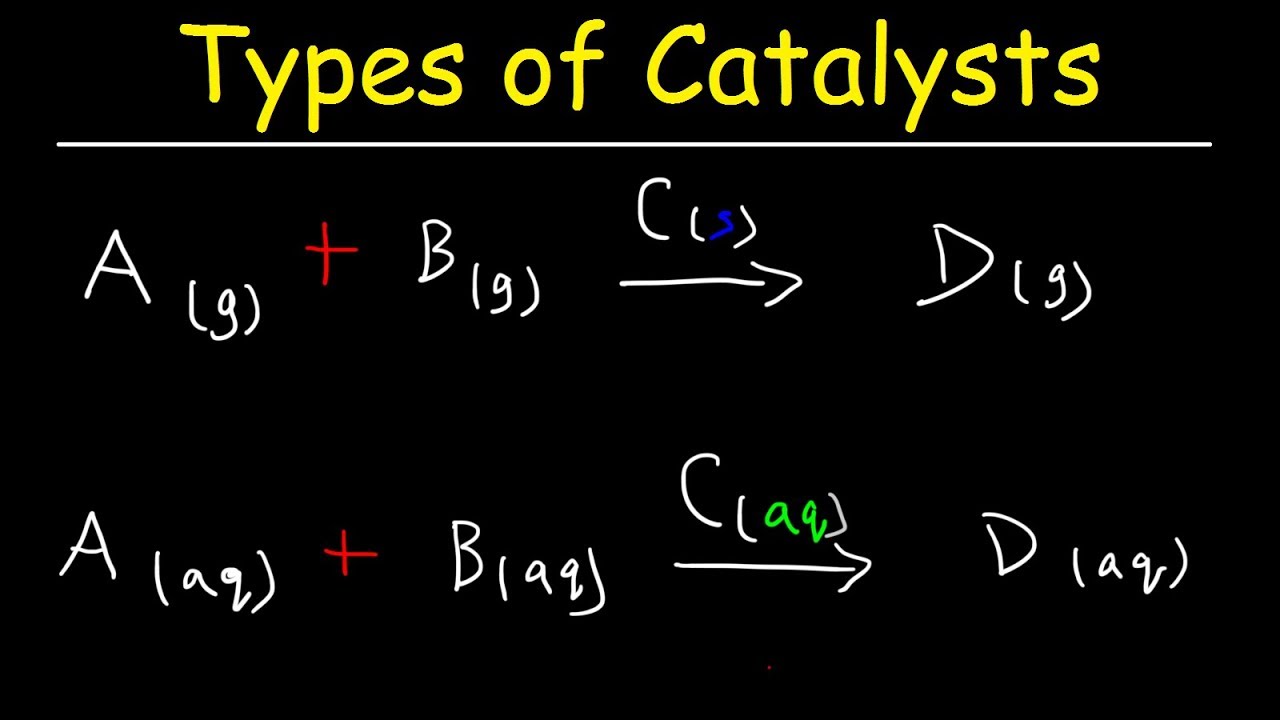
Catalysis Equation . Heterogeneous catalysts, homogeneous catalysts, and enzymes. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In this section, we will examine the three major classes of catalysts: Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between the three principal classes of catalysts. For base catalysis there are two possible situations 1. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts can be homogenous (in the same phase as the reactants) or. Estimate the activation energy for each process, and identify which one involves a catalyst. Activation energies are calculated by subtracting the. Activation energies are calculated by subtracting the.
from www.youtube.com
The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Estimate the activation energy for each process, and identify which one involves a catalyst. Estimate the activation energy for each process, and identify which one involves a catalyst. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Heterogeneous catalysts, homogeneous catalysts, and enzymes. For base catalysis there are two possible situations 1. In this section, we will examine the three major classes of catalysts: Activation energies are calculated by subtracting the. Describe the similarities and differences between the three principal classes of catalysts.
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube
Catalysis Equation Describe the similarities and differences between the three principal classes of catalysts. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will examine the three major classes of catalysts: For base catalysis there are two possible situations 1. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Describe the similarities and differences between the three principal classes of catalysts. Activation energies are calculated by subtracting the. Estimate the activation energy for each process, and identify which one involves a catalyst. Activation energies are calculated by subtracting the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Estimate the activation energy for each process, and identify which one involves a catalyst. Catalysts can be homogenous (in the same phase as the reactants) or.
From www.researchgate.net
Catalysis reaction Reaction of Cu(i)Lcatalysed Catalysis Equation Describe the similarities and differences between the three principal classes of catalysts. For base catalysis there are two possible situations 1. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Activation energies are calculated by subtracting the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Estimate the activation energy for each process, and. Catalysis Equation.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalysis Equation Activation energies are calculated by subtracting the. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Describe the similarities and differences between the three principal classes of catalysts. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Estimate the activation energy for. Catalysis Equation.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysis Equation Activation energies are calculated by subtracting the. In this section, we will examine the three major classes of catalysts: The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts can be homogenous (in the same phase as the reactants) or. Heterogeneous catalysts, homogeneous catalysts, and enzymes. For base catalysis there. Catalysis Equation.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalysis Equation Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will examine the three major classes of catalysts: Catalysts can be homogenous (in the same phase as the reactants) or. Activation energies are calculated by subtracting the. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Estimate the activation energy. Catalysis Equation.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalysis Equation Catalysts can be homogenous (in the same phase as the reactants) or. Heterogeneous catalysts, homogeneous catalysts, and enzymes. For base catalysis there are two possible situations 1. Activation energies are calculated by subtracting the. Estimate the activation energy for each process, and identify which one involves a catalyst. In this section, we will examine the three major classes of catalysts:. Catalysis Equation.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3206932 Catalysis Equation Describe the similarities and differences between the three principal classes of catalysts. Activation energies are calculated by subtracting the. Catalysts can be homogenous (in the same phase as the reactants) or. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Activation energies are calculated by subtracting the. For base catalysis there are two possible situations 1. The brønsted catalysis equation or law of. Catalysis Equation.
From www.slideserve.com
PPT Catalytic Converter PowerPoint Presentation ID6558391 Catalysis Equation Estimate the activation energy for each process, and identify which one involves a catalyst. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Estimate the activation energy for each process, and identify which one involves a catalyst. Activation energies. Catalysis Equation.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysis Equation For base catalysis there are two possible situations 1. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Estimate the activation energy for each process, and identify which one involves a catalyst. Estimate the activation energy for each process, and identify which one involves a catalyst. Heterogeneous catalysts, homogeneous catalysts,. Catalysis Equation.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalysis Equation Catalysts can be homogenous (in the same phase as the reactants) or. Describe the similarities and differences between the three principal classes of catalysts. For base catalysis there are two possible situations 1. Heterogeneous catalysts, homogeneous catalysts, and enzymes. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Estimate the. Catalysis Equation.
From www.researchgate.net
Dimensionless equations for catalytic reactions in discretized terms Catalysis Equation In this section, we will examine the three major classes of catalysts: Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between the three principal classes of catalysts. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. For base catalysis. Catalysis Equation.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Catalysis Equation Catalysts can be homogenous (in the same phase as the reactants) or. In this section, we will examine the three major classes of catalysts: Activation energies are calculated by subtracting the. Describe the similarities and differences between the three principal classes of catalysts. Estimate the activation energy for each process, and identify which one involves a catalyst. For base catalysis. Catalysis Equation.
From www.numerade.com
SOLVEDIdentify the catalyst in each equation. Catalysis Equation Activation energies are calculated by subtracting the. Catalysts can be homogenous (in the same phase as the reactants) or. In this section, we will examine the three major classes of catalysts: Estimate the activation energy for each process, and identify which one involves a catalyst. Estimate the activation energy for each process, and identify which one involves a catalyst. For. Catalysis Equation.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysis Equation Activation energies are calculated by subtracting the. Estimate the activation energy for each process, and identify which one involves a catalyst. Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between the three principal classes of catalysts. In this section, we will examine the three major classes of catalysts: Heterogeneous. Catalysis Equation.
From brainly.in
define catalyst with example ?? Brainly.in Catalysis Equation The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Estimate the activation energy for each process, and identify which one involves a catalyst. Activation energies are calculated by subtracting the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide. Catalysis Equation.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalysis Equation The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts can be homogenous (in the same phase as the reactants) or. Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between the three principal classes of catalysts. Activation energies are. Catalysis Equation.
From www.chemistrystudent.com
Homogeneous Catalysis (ALevel) ChemistryStudent Catalysis Equation Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between the three principal classes of catalysts. Activation energies are calculated by subtracting the. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Activation energies are calculated by subtracting the. In. Catalysis Equation.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysis Equation Heterogeneous catalysts, homogeneous catalysts, and enzymes. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Estimate the activation energy for each process, and identify which one involves a catalyst. In this section, we will examine the three major classes of catalysts: Activation energies are calculated by subtracting the. Activation energies. Catalysis Equation.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalysis Equation The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Estimate the activation energy for each process, and identify which one. Catalysis Equation.
From www.youtube.com
Michaelis Menten Equation ENZYME CATALYSIS BIOCHEMICAL REACTION Catalysis Equation Estimate the activation energy for each process, and identify which one involves a catalyst. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Describe the similarities and differences between the three principal classes of catalysts. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Activation energies are calculated by subtracting the. Catalysts can be. Catalysis Equation.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysis Equation Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Activation energies are calculated by subtracting the. In this section, we will examine the three major classes of catalysts: For base catalysis there are two possible situations 1. Activation energies are calculated by subtracting the. Describe the similarities and differences between the. Catalysis Equation.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalysis Equation Catalysts can be homogenous (in the same phase as the reactants) or. Activation energies are calculated by subtracting the. For base catalysis there are two possible situations 1. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Activation energies are calculated by subtracting the. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In. Catalysis Equation.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysis Equation Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between the three principal classes of catalysts. Activation energies are calculated by subtracting the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In this section, we will examine the three major. Catalysis Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalysis Equation Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Describe the similarities and differences between the three principal classes of catalysts. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. Activation energies are calculated by subtracting the. Activation energies are calculated by. Catalysis Equation.
From www.youtube.com
01.04 Brønsted Acid Catalysis YouTube Catalysis Equation Estimate the activation energy for each process, and identify which one involves a catalyst. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts can be homogenous (in the same phase as the reactants) or. In this section, we will examine the three major classes of catalysts: Activation energies are. Catalysis Equation.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID409487 Catalysis Equation Activation energies are calculated by subtracting the. Estimate the activation energy for each process, and identify which one involves a catalyst. For base catalysis there are two possible situations 1. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted,. Catalysis Equation.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalysis Equation The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. Catalysts can be homogenous (in the same phase as the reactants) or. Activation energies are calculated by subtracting the. Estimate the activation energy. Catalysis Equation.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalysis Equation The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Estimate the activation energy for each process, and identify which one involves a catalyst. In this section, we will examine the three major classes of catalysts: Activation energies are calculated by subtracting the. Catalysts can be homogenous (in the same phase. Catalysis Equation.
From www.researchgate.net
The laws and equations of catalysis and electrolysis derive from one Catalysis Equation Describe the similarities and differences between the three principal classes of catalysts. Estimate the activation energy for each process, and identify which one involves a catalyst. In this section, we will examine the three major classes of catalysts: Activation energies are calculated by subtracting the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a. Catalysis Equation.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalysis Equation Estimate the activation energy for each process, and identify which one involves a catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. For base catalysis there are two possible situations 1. Activation energies are calculated by subtracting the. Catalysts can be homogenous (in the same phase as the reactants) or. Activation energies are calculated by subtracting the. Estimate the activation energy for. Catalysis Equation.
From ar.inspiredpencil.com
Catalyst Equation Catalysis Equation Catalysts can be homogenous (in the same phase as the reactants) or. Heterogeneous catalysts, homogeneous catalysts, and enzymes. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Estimate the activation energy for. Catalysis Equation.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalysis Equation Describe the similarities and differences between the three principal classes of catalysts. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. For base catalysis there are two possible situations 1. Estimate the. Catalysis Equation.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalysis Equation Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Describe the similarities and differences between the three principal classes of catalysts. Catalysts can be homogenous (in the same phase as the reactants). Catalysis Equation.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysis Equation In this section, we will examine the three major classes of catalysts: Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. Describe the similarities and differences between the three principal classes of catalysts. Activation energies are calculated by subtracting. Catalysis Equation.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalysis Equation Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The brønsted catalysis equation or law of correlation, after johannes nicolaus brønsted, gives the relationship between acid strength and. Catalysts can be homogenous (in the same phase as the reactants) or. Estimate the activation energy for each process, and identify which one. Catalysis Equation.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalysis Equation Activation energies are calculated by subtracting the. Activation energies are calculated by subtracting the. In this section, we will examine the three major classes of catalysts: Catalysts can be homogenous (in the same phase as the reactants) or. Describe the similarities and differences between the three principal classes of catalysts. Estimate the activation energy for each process, and identify which. Catalysis Equation.