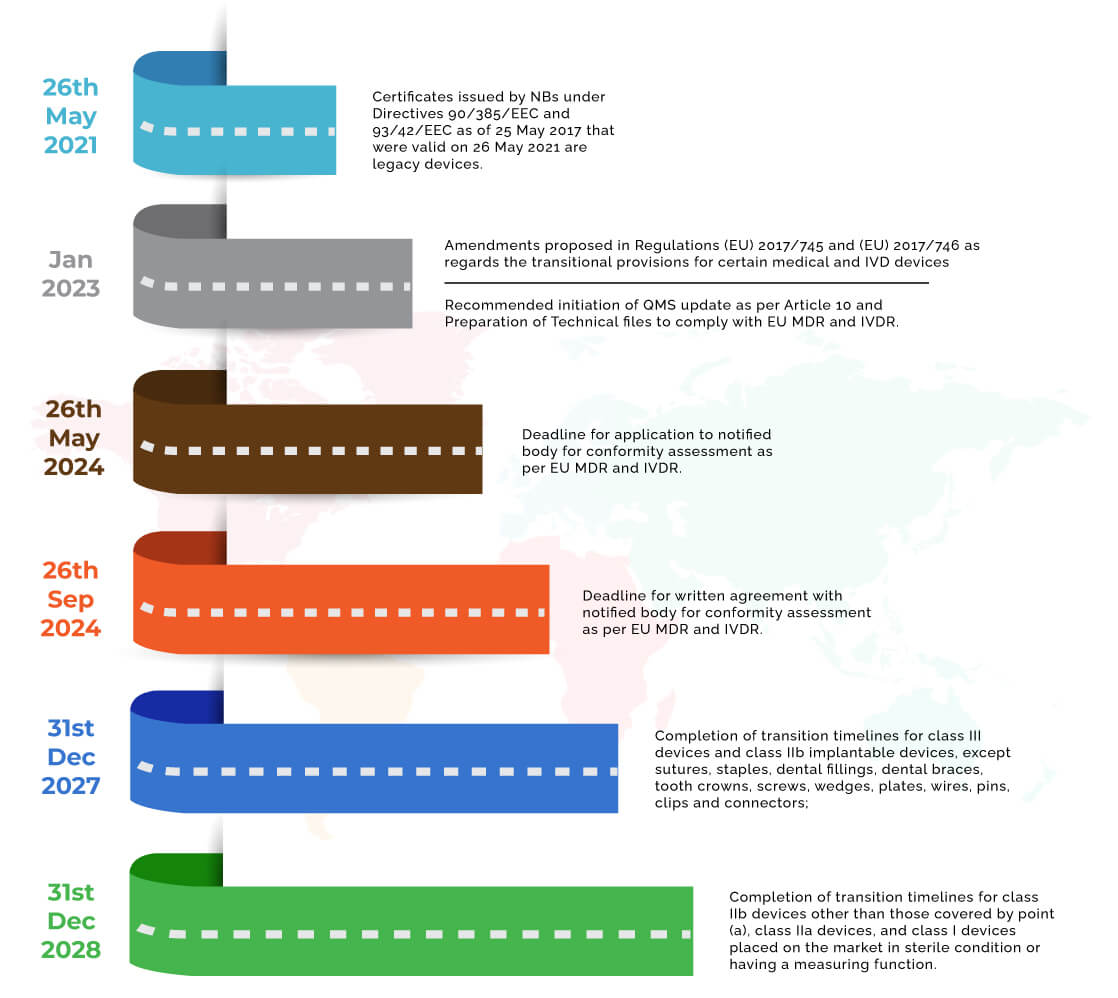
Medical Device Regulations Eu 2017 . Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the.
from www.vrogue.co
Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu.
Eu Mdr Medical Device Regulations Timeline vrogue.co
Medical Device Regulations Eu 2017 With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing.
From www.hpcosmos.com
Certificates based on MDR Medical Device Regulation (EU) 2017/745 and Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Regulation. Medical Device Regulations Eu 2017.
From www.dlrcgroup.com
Impact of Article 117 of the EU Medical Device Regulations 2017/745 DLRC Medical Device Regulations Eu 2017 With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The medical device regulation (mdr), which was adopted in april 2017, changes the. Medical Device Regulations Eu 2017.
From www.presentationeze.com
MDR Medical Device Regulation EU 2017 745 Timeline PresentationEZE Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of. Medical Device Regulations Eu 2017.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Regulations Eu 2017 The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical.. Medical Device Regulations Eu 2017.
From www.presentationeze.com
MDR 2017 745 Timeline. Implementation of the Medical Device Regulation Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal. Medical Device Regulations Eu 2017.
From www.vrogue.co
Eu Mdr Medical Device Regulations Timeline vrogue.co Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed. Medical Device Regulations Eu 2017.
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Medical Device Regulations Eu 2017 The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro. Medical Device Regulations Eu 2017.
From planetinnovation.com
The EU Medical Device Regulations (EU MDR 2017/745) in a nutshell Medical Device Regulations Eu 2017 The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic. Medical Device Regulations Eu 2017.
From www.qualitymeddev.com
Cybersecurity Requirements for Medical Devices and EU MDR 2017/745 Medical Device Regulations Eu 2017 The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal. Medical Device Regulations Eu 2017.
From www.auxergo.com
The Interactive Guide Under The New EU Regulations on Medical Devices Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation. Medical Device Regulations Eu 2017.
From www.jamasoftware.com
What the New Medical Device Regulations (EU MDR) Mean for You Jama Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april. Medical Device Regulations Eu 2017.
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Medical Device Regulations Eu 2017 The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The medical device regulation (mdr), which was adopted in april 2017, changes the european. Medical Device Regulations Eu 2017.
From crfweb.com
Medical Device Regulations Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices. Medical Device Regulations Eu 2017.
From happeningnext.com
European Medical Devices Regulation (EU) 2017/745 Online April 18 Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation. Medical Device Regulations Eu 2017.
From www.iascertification.com
EUMDR Certification Medical Device Regulation IAS Medical Device Regulations Eu 2017 The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices. Medical Device Regulations Eu 2017.
From www.researchgate.net
(PDF) The New European Medical Device Regulation 2017/745 Main Changes Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical.. Medical Device Regulations Eu 2017.
From www.pinterest.com
Regulation (EU) 2017/745 on medical devices Medical device Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of. Medical Device Regulations Eu 2017.
From omcmedical.com
Vigilance Terms & Concepts (EU) 2017/745 on Medical Devices Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and. Medical Device Regulations Eu 2017.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulations Eu 2017 With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Medical Device Regulations Eu 2017.
From www.tuv.com
EU Medical Device Regulation MDR 2017/745 WO TÜV Rheinland Medical Device Regulations Eu 2017 With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/745 of the european parliament and of the council of 5. Medical Device Regulations Eu 2017.
From www.regulatoryrapporteur.org
One year experience on EU Medical Devices Regulation (MDR) 2017/745 for Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of. Medical Device Regulations Eu 2017.
From compliantmd.com
Introduction to the Medical Device Regulation (EU) 2017/745 CompliantMD Medical Device Regulations Eu 2017 The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation. Medical Device Regulations Eu 2017.
From www.tuv.com
EU Medical Device Regulation MDR 2017/745 WO TÜV Rheinland Medical Device Regulations Eu 2017 The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the.. Medical Device Regulations Eu 2017.
From www.universalmedica.com
Key Aspects of New EU Medical Devices Regulation (EU 2017/745 Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Medical Device Regulations Eu 2017.
From connectorsupplier.com
EU MDR Update to Medical Device Regulations in Europe Medical Device Regulations Eu 2017 The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of. Medical Device Regulations Eu 2017.
From globalpccs.com
EU Medical Device Regulation Compliance Services in IMDS CDX ELV Medical Device Regulations Eu 2017 The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. With. Medical Device Regulations Eu 2017.
From www.eucope.org
MDR/IVDR Implementation EC’s Proposal on 6 January 2023 Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of. Medical Device Regulations Eu 2017.
From keyruslifescience.com
Accelerate the compliance process of your product portfolio in line Medical Device Regulations Eu 2017 The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic. Medical Device Regulations Eu 2017.
From academycenters.com
Medical Device Regulation Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of. Medical Device Regulations Eu 2017.
From gxp-training.com
Medical Device Regulation MDR 2017/745 Course and Certificate Medical Device Regulations Eu 2017 The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Medical Device Regulations Eu 2017.
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Medical Device Regulations Eu 2017 The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices. Medical Device Regulations Eu 2017.
From www.researchgate.net
(PDF) A Review on European Union New Medical Device Regulations2017 Medical Device Regulations Eu 2017 With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. The medical device regulation (mdr), which was adopted in april 2017, changes the. Medical Device Regulations Eu 2017.
From pdfprof.com
Medical Device Regulation (EU 2017/745) Conformity Assessment Medical Device Regulations Eu 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the. Medical Device Regulations Eu 2017.
From www.linkedin.com
Article 117 of the Medical Device Regulation (EU MDR) 2017/745 Medical Device Regulations Eu 2017 The european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation. Medical Device Regulations Eu 2017.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (IN) Medical Device Regulations Eu 2017 Regulation (eu) 2017/746 of the european parliament and of the council of 5 april 2017 on in vitro diagnostic medical devices and repealing. With effect from 26 may 2021, regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices. The european commission published the first implementing regulation related to the eu mdr. Medical Device Regulations Eu 2017.