Fe D Electrons . 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. It’s a transition metal from. Iron is in group 8, so. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. An element’s valency is the number of electrons it. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The δ splitting energy for tetrahedral metal complexes (four ligands), δ. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.
from www.chegg.com
It’s a transition metal from. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. An element’s valency is the number of electrons it. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. Iron is in group 8, so. The chemical element iron has the atomic number 26 and the symbol fe (from latin:
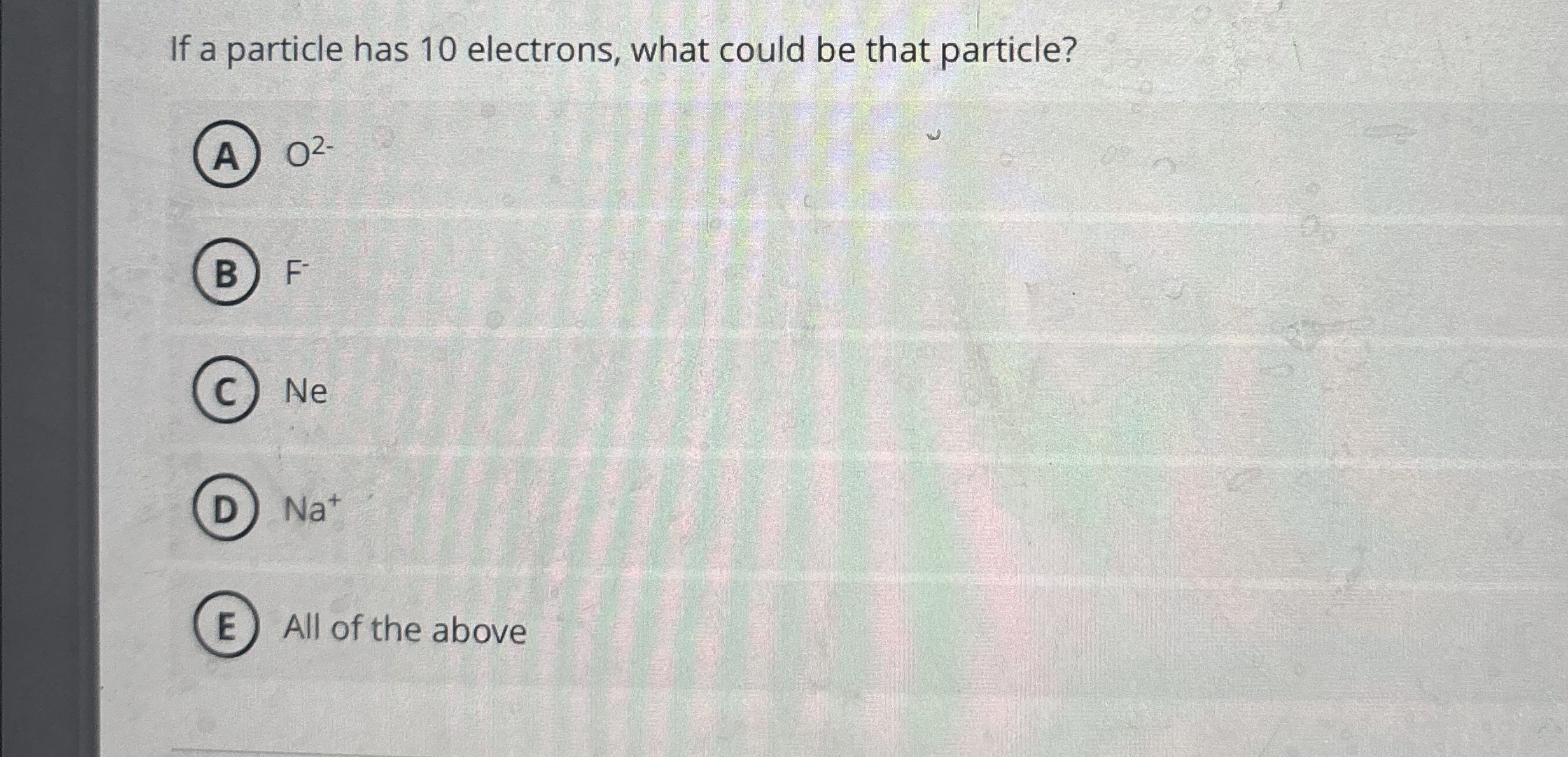
Solved If a particle has 10 electrons, what could be that
Fe D Electrons 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. An element’s valency is the number of electrons it. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. It’s a transition metal from. The chemical element iron has the atomic number 26 and the symbol fe (from latin: This handy electron configuration chart compiles the electron configurations of the elements up through number 104. Iron is in group 8, so. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. The δ splitting energy for tetrahedral metal complexes (four ligands), δ.
From sciencenotes.org
List of Electron Configurations of Elements Fe D Electrons The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. It’s a transition metal from. The chemical element iron has the atomic number 26 and the. Fe D Electrons.
From www.chegg.com
Solved In covalent bonds, valence electrons are shared Fe D Electrons The chemical element iron has the atomic number 26 and the symbol fe (from latin: An element’s valency is the number of electrons it. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. This handy electron configuration chart compiles the electron configurations of the elements up through number 104.. Fe D Electrons.
From pressbooks.bccampus.ca
1.6 Periodic Variations in Element Properties Chemistry for Fe D Electrons It’s a transition metal from. Iron is in group 8, so. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. An element’s valency is the number of electrons it. The aufbau principle explains. Fe D Electrons.
From www.gauthmath.com
Solved When will an atom give up electrons? when the n=2 level is full Fe D Electrons This handy electron configuration chart compiles the electron configurations of the elements up through number 104. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is in group 8, so. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. The aufbau principle explains how electrons fill low energy orbitals (closer to. Fe D Electrons.
From www.youtube.com
Chimie 1 Atomistique Électrons de valence et de cœur (Leçon 6) YouTube Fe D Electrons It’s a transition metal from. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. An element’s valency is the number of electrons it. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. Iron is in group 8, so. 107 rows the electron configuration. Fe D Electrons.
From www.vedantu.com
Fe{{F}_{6}}]}^{3}} has Fe atom _________hybridized with unpaired Fe D Electrons It’s a transition metal from. The chemical element iron has the atomic number 26 and the symbol fe (from latin: 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. The aufbau principle explains how electrons fill low energy orbitals (closer to. Fe D Electrons.
From fr.wikihow.com
Comment déterminer le nombre d’électrons de valence Fe D Electrons The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. Iron. Fe D Electrons.
From www.researchgate.net
Configurations of the 3d electrons of Fe(II) and Fe(III), their Fe D Electrons This handy electron configuration chart compiles the electron configurations of the elements up through number 104. Iron is in group 8, so. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. How to write the electron configuration. Fe D Electrons.
From valenceelectrons.com
Iron(Fe) electron configuration and orbital diagram Fe D Electrons Iron is in group 8, so. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. An element’s valency is the number of electrons it. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. Valence. Fe D Electrons.
From www.chegg.com
Solved The numbers of electrons in a chlorine atom's first Fe D Electrons The δ splitting energy for tetrahedral metal complexes (four ligands), δ. The chemical element iron has the atomic number 26 and the symbol fe (from latin: 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. This handy electron configuration chart compiles. Fe D Electrons.
From socratic.org
How many unpaired electrons are in an atom of Co in its ground state Fe D Electrons This handy electron configuration chart compiles the electron configurations of the elements up through number 104. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. It’s a transition metal from. An element’s valency is the number of electrons it. The aufbau principle explains how electrons. Fe D Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Fe D Electrons It’s a transition metal from. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. An element’s valency is the number of electrons it. This handy. Fe D Electrons.
From www.toppr.com
The number of d electrons in Fe^2 + (Z = 26) is not equal to the Fe D Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. Iron is in group 8, so. An element’s valency is the number of electrons it. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. It’s. Fe D Electrons.
From www.toppr.com
The number of d electrons in Fe^2 + (Z = 26) is not equal to the Fe D Electrons It’s a transition metal from. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. An element’s valency is the number of electrons it.. Fe D Electrons.
From www.numerade.com
SOLVED18. Write out the electron configuration for Fe" , Fe'? and Fe Fe D Electrons This handy electron configuration chart compiles the electron configurations of the elements up through number 104. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. An element’s valency is the number of electrons it. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. How to write. Fe D Electrons.
From www.numerade.com
SOLVEDWrite full electron configurations and indicate the valence Fe D Electrons 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. An element’s valency is the number of electrons it. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. The chemical element iron has the atomic. Fe D Electrons.
From www.youtube.com
Valence Electrons & Electron Configurations YouTube Fe D Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from. Valence electrons are a single outer shell electron in an atom that is responsible for the. Fe D Electrons.
From www.clipartkey.com
Clip Art File With Unpaired Electrons Periodic Table Of Molecules Fe D Electrons The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is in group 8, so. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of. Fe D Electrons.
From www.numerade.com
SOLVEDThe number of delectrons retained in Fe^2+ (At. number of Fe=26 Fe D Electrons Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. It’s a transition metal from. This handy electron configuration chart compiles the electron configurations of the elements up through. Fe D Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Fe D Electrons The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. 107 rows the electron configuration of an atom of any element is the of electrons per. Fe D Electrons.
From csmetrics.org
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions) 最も Fe D Electrons 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. The δ splitting energy for tetrahedral metal complexes. Fe D Electrons.
From www.toppr.com
Electronic Configuration of Iron Fe element Valency, Applications Fe D Electrons The δ splitting energy for tetrahedral metal complexes (four ligands), δ. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. An element’s valency is the number of electrons it. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy. Fe D Electrons.
From ar.inspiredpencil.com
Electron Subshells Fe D Electrons The chemical element iron has the atomic number 26 and the symbol fe (from latin: The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. It’s a transition metal from. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. An element’s valency is. Fe D Electrons.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube Fe D Electrons Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. An element’s valency is the number of electrons it. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. The chemical element iron has the. Fe D Electrons.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Fe D Electrons An element’s valency is the number of electrons it. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. This handy. Fe D Electrons.
From www.chegg.com
Solved N for these represent the number of unpaired Fe D Electrons This handy electron configuration chart compiles the electron configurations of the elements up through number 104. An element’s valency is the number of electrons it. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. Valence electrons are a single outer shell. Fe D Electrons.
From socratic.org
Which following pairs of atoms, have a lower electron affinity? a) Ca,K Fe D Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.. Fe D Electrons.
From www.chegg.com
Solved If a particle has 10 electrons, what could be that Fe D Electrons Iron is in group 8, so. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. It’s a transition metal from. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. How to write the electron. Fe D Electrons.
From www.chegg.com
Solved The final acceptor of electrons during oxidative Fe D Electrons An element’s valency is the number of electrons it. It’s a transition metal from. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher. Fe D Electrons.
From www.toppr.com
The number of d electrons in Fe^2 + (Z = 26 ) is not equal to the Fe D Electrons The δ splitting energy for tetrahedral metal complexes (four ligands), δ. It’s a transition metal from. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. The chemical element iron has the atomic number 26 and the symbol fe (from latin: How. Fe D Electrons.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Fe D Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the. Fe D Electrons.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Fe D Electrons It’s a transition metal from. The δ splitting energy for tetrahedral metal complexes (four ligands), δ. Iron is in group 8, so. This handy electron configuration chart compiles the electron configurations of the elements up through number 104. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of. Fe D Electrons.
From www.youtube.com
The number of delectrons retained in \( \mathrm{Fe}^{2+} \) (At. n Fe D Electrons 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. An element’s valency is the number of electrons it. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. Fe D Electrons.
From www.chegg.com
Solved What is the delectron count for the following a. Fe D Electrons Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. It’s a transition metal from. Iron is in group 8, so. 107 rows the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state.. Fe D Electrons.
From www.numerade.com
The correct order of number of unpaired electrons in the ion Cu^2+, Ni Fe D Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. The chemical element iron has the atomic number 26 and the symbol fe (from latin:. Fe D Electrons.