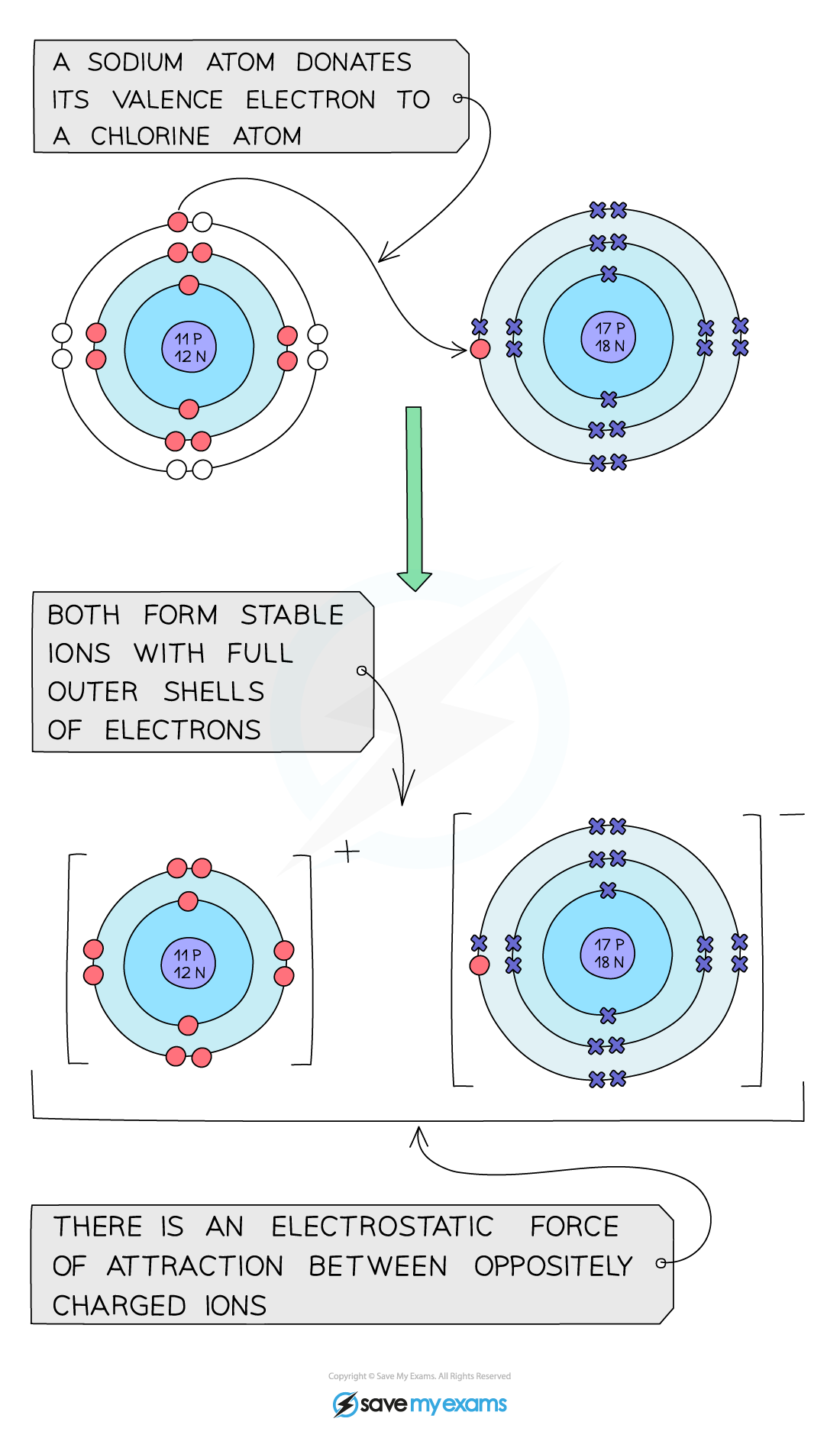
Are Ionic Bonds Lustrous . The ions are in fixed positions in the lattice. Ionic compounds are poor conductors in the solid state. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Solutions of ionic compounds and. Solutions of ionic compounds and melted ionic compounds conduct. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Covalent bonds are highly stable bonds with. Ionic compounds are hard and brittle. What is an ionic bond? Most ionic solids, however, dissolve. The ions may either be monatomic or polyatomic. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a.
from www.linstitute.net
Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct. Ionic compounds are hard and brittle. Covalent bonds are highly stable bonds with. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. What is an ionic bond? The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Most ionic solids, however, dissolve. Ionic compounds have high melting points.
Edexcel IGCSE Chemistry 复习笔记 1.6 4 Ionic Bonds Dot & Cross Diagrams翰林国际教育
Are Ionic Bonds Lustrous Covalent bonds are highly stable bonds with. They are therefore unable to move and carry a charge. Solutions of ionic compounds and melted ionic compounds conduct. The ions may either be monatomic or polyatomic. What is an ionic bond? Most ionic solids, however, dissolve. Ionic compounds are hard and brittle. Ionic compounds are poor conductors in the solid state. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Solutions of ionic compounds and. Covalent bonds are highly stable bonds with. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 4 Ionic Bonds Dot & Cross Diagrams翰林国际教育 Are Ionic Bonds Lustrous Solutions of ionic compounds and melted ionic compounds conduct. Most ionic solids, however, dissolve. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Ionic compounds have high melting points. Ionic compounds tend to be crystalline structures with high melting points that are. Are Ionic Bonds Lustrous.
From www.dreamstime.com
Ionic Bond and Electrostatic Attraction from Chemical Bonding Outline Are Ionic Bonds Lustrous Covalent bonds are highly stable bonds with. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The ions may either be monatomic or polyatomic. Ionic compounds are hard and brittle. Ionic compounds are hard and brittle. They are therefore unable to move and carry a charge. Ionic compounds are poor conductors in the solid. Are Ionic Bonds Lustrous.
From slideplayer.com
Chemical Formulas and Chemical Bonding ppt download Are Ionic Bonds Lustrous Ionic compounds are hard and brittle. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Ionic compounds are hard and brittle. Ionic compounds tend to. Are Ionic Bonds Lustrous.
From slideplayer.com
“Dancer”, Fernando Botero Colombian, ppt download Are Ionic Bonds Lustrous Solutions of ionic compounds and. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Covalent bonds are highly stable bonds with. Ionic compounds are hard and brittle. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds are poor conductors in the solid state. What is an. Are Ionic Bonds Lustrous.
From slideplayer.com
Chemical Bonds Notes 10/16/ ppt download Are Ionic Bonds Lustrous Most ionic solids, however, dissolve. Ionic compounds have high melting points. Solutions of ionic compounds and. Solutions of ionic compounds and melted ionic compounds conduct. The ions are in fixed positions in the lattice. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds are poor conductors in the solid state. An ionic. Are Ionic Bonds Lustrous.
From www.savemyexams.com
Electronegativity & Bonding CIE A Level Chemistry Revision Notes 2025 Are Ionic Bonds Lustrous Solutions of ionic compounds and. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Most ionic solids, however, dissolve. Ionic compounds have high melting points. They are therefore unable to move and carry a charge. Ionic bond, type. Are Ionic Bonds Lustrous.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Are Ionic Bonds Lustrous What is an ionic bond? Ionic compounds are hard and brittle. Solutions of ionic compounds and. Ionic compounds dissociate into ions when dissolved in water. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The ions are in fixed positions in the lattice. Ionic compounds tend to be crystalline structures with high. Are Ionic Bonds Lustrous.
From informacionpublica.svet.gob.gt
Types Of Chemical Bonds What Are Chemical Bonds Covalent Are Ionic Bonds Lustrous Solutions of ionic compounds and. The ions are in fixed positions in the lattice. What is an ionic bond? Ionic compounds have high melting points. The ions may either be monatomic or polyatomic. Ionic compounds dissociate into ions when dissolved in water. They are therefore unable to move and carry a charge. Solutions of ionic compounds and melted ionic compounds. Are Ionic Bonds Lustrous.
From ar.inspiredpencil.com
Metallic Bonds Are Ionic Bonds Lustrous Most ionic solids, however, dissolve. Solutions of ionic compounds and. The ions may either be monatomic or polyatomic. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Ionic compounds are hard and brittle. What is an ionic bond? Ionic compounds are poor conductors in the solid state. The ions are in fixed positions. Are Ionic Bonds Lustrous.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Are Ionic Bonds Lustrous Covalent bonds are highly stable bonds with. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic compounds are hard and brittle. Ionic compounds are poor conductors in the solid state. Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct. Ionic compounds dissociate into ions when. Are Ionic Bonds Lustrous.
From slideplayer.com
Chemical Bonding. ppt download Are Ionic Bonds Lustrous Solutions of ionic compounds and melted ionic compounds conduct. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. Covalent bonds are highly stable bonds with. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Most ionic solids, however, dissolve. Solutions of ionic compounds and. What is. Are Ionic Bonds Lustrous.
From h-o-m-e.org
Ionic Bond Understanding the Force of Attraction Are Ionic Bonds Lustrous An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. They are therefore unable to move and carry a charge. Ionic compounds are poor conductors in the. Are Ionic Bonds Lustrous.
From www.vedantu.com
Exploring the Key Difference between ionic covalent and metallic bonds Are Ionic Bonds Lustrous Ionic compounds are poor conductors in the solid state. Solutions of ionic compounds and melted ionic compounds conduct. Solutions of ionic compounds and. Ionic compounds dissociate into ions when dissolved in water. Most ionic solids, however, dissolve. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The ions may either be monatomic. Are Ionic Bonds Lustrous.
From slideplayer.com
The Nature of Matter. ppt download Are Ionic Bonds Lustrous Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic compounds are hard and brittle. The ions may either be monatomic or polyatomic. Ionic compounds are hard and brittle. Covalent bonds are highly stable bonds with. Solutions. Are Ionic Bonds Lustrous.
From byjus.com
Is the ionic bond more strong than covalent bond Are Ionic Bonds Lustrous Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Most ionic solids, however, dissolve. What is an ionic bond? The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Ionic compounds dissociate into ions when dissolved in water. Covalent bonds are highly stable bonds with. Ionic compounds are. Are Ionic Bonds Lustrous.
From www.youtube.com
How to tell if Ionic Bond or Covalent Bond YouTube Are Ionic Bonds Lustrous Ionic compounds are hard and brittle. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic compounds are poor conductors in the solid state. Solutions of ionic compounds and melted ionic compounds conduct. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. The ions are in. Are Ionic Bonds Lustrous.
From slideplayer.com
INTRODUCTION TO BIOCHEMISTRY ppt download Are Ionic Bonds Lustrous They are therefore unable to move and carry a charge. Solutions of ionic compounds and melted ionic compounds conduct. Ionic compounds dissociate into ions when dissolved in water. Most ionic solids, however, dissolve. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The ions may either be monatomic or polyatomic. Ionic compounds. Are Ionic Bonds Lustrous.
From www.scribd.com
Ionic Bond PDF Ion Chemical Compounds Are Ionic Bonds Lustrous Ionic compounds are hard and brittle. Ionic compounds have high melting points. What is an ionic bond? The ions may either be monatomic or polyatomic. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Ionic compounds are poor conductors in the solid. Are Ionic Bonds Lustrous.
From byjus.com
Sodium was found to have a strong tendency to form Na+ during ionic Are Ionic Bonds Lustrous Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Most ionic solids, however, dissolve. They are therefore unable to move and carry a charge. Ionic compounds are hard and brittle. The ions are in fixed positions. Are Ionic Bonds Lustrous.
From edu.rsc.org
How to teach ionic bonding at 1416 CPD RSC Education Are Ionic Bonds Lustrous The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Covalent bonds are highly stable bonds with. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Solutions of ionic compounds and melted ionic compounds conduct. Ionic. Are Ionic Bonds Lustrous.
From slideplayer.com
Ionic Bonding. ppt download Are Ionic Bonds Lustrous Solutions of ionic compounds and. Ionic compounds are poor conductors in the solid state. The ions may either be monatomic or polyatomic. Ionic compounds have high melting points. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. What is an ionic bond? They are therefore unable to move and carry a charge. Ionic. Are Ionic Bonds Lustrous.
From slideplayer.com
UNIT 5 Patterns and Compounds ppt download Are Ionic Bonds Lustrous Ionic compounds are hard and brittle. Ionic compounds are poor conductors in the solid state. Solutions of ionic compounds and melted ionic compounds conduct. Ionic compounds have high melting points. Most ionic solids, however, dissolve. Ionic compounds dissociate into ions when dissolved in water. The ions are in fixed positions in the lattice. Covalent bonds are highly stable bonds with.. Are Ionic Bonds Lustrous.
From slideplayer.com
Ionic Bonding/Naming. ppt download Are Ionic Bonds Lustrous Most ionic solids, however, dissolve. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Solutions of ionic compounds and. The ions are in fixed positions in the lattice. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The ions may either be. Are Ionic Bonds Lustrous.
From slideplayer.com
ppt download Are Ionic Bonds Lustrous They are therefore unable to move and carry a charge. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Covalent bonds. Are Ionic Bonds Lustrous.
From slideplayer.com
WarmUp 10/17/16 Name the difference between an ionic and covalent Are Ionic Bonds Lustrous Covalent bonds are highly stable bonds with. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Most ionic solids, however, dissolve. What is an ionic bond? The. Are Ionic Bonds Lustrous.
From slideplayer.com
Bonding. ppt download Are Ionic Bonds Lustrous Ionic compounds are hard and brittle. Most ionic solids, however, dissolve. What is an ionic bond? Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Ionic compounds are hard and brittle. The ions are in fixed. Are Ionic Bonds Lustrous.
From sailsojourn.com
Ionic Bond Definition, Types, Properties, Examples (2022) Are Ionic Bonds Lustrous Most ionic solids, however, dissolve. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Solutions of ionic compounds and. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. They are therefore unable to move and. Are Ionic Bonds Lustrous.
From slideplayer.com
Bellringer Answer the following questions ppt download Are Ionic Bonds Lustrous Covalent bonds are highly stable bonds with. Ionic compounds are hard and brittle. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. What is an ionic bond? The ions may either be monatomic or polyatomic. Ionic compounds are hard and brittle. Most ionic solids, however, dissolve. An ionic bond, also known as. Are Ionic Bonds Lustrous.
From slideplayer.com
Chemical Bonding. ppt download Are Ionic Bonds Lustrous Solutions of ionic compounds and. The ions are in fixed positions in the lattice. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Most ionic solids, however, dissolve. They are therefore unable to move and carry a charge. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Are Ionic Bonds Lustrous.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Are Ionic Bonds Lustrous Most ionic solids, however, dissolve. Ionic compounds are hard and brittle. Ionic compounds have high melting points. Ionic compounds dissociate into ions when dissolved in water. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic compounds are hard and brittle. Ionic compounds are poor conductors in the solid state. The reason. Are Ionic Bonds Lustrous.
From evulpo.com
evulpo Ionic bonding Are Ionic Bonds Lustrous What is an ionic bond? Ionic compounds are hard and brittle. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Ionic compounds are poor conductors in the solid state. An ionic bond, also known as an electrovalent bond,. Are Ionic Bonds Lustrous.
From nathanaelancefarley.blogspot.com
Which of the Following Best Describes an Ionic Bond NathanaelanceFarley Are Ionic Bonds Lustrous Ionic compounds are hard and brittle. Ionic compounds are hard and brittle. Solutions of ionic compounds and. What is an ionic bond? The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds tend to be crystalline structures with high melting points that are. Are Ionic Bonds Lustrous.
From slideplayer.com
Ionic Charges The outer electrons on an atom are called Valence Are Ionic Bonds Lustrous The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. What is an ionic bond? Ionic compounds are hard and brittle. Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct. They are therefore unable to move and carry a charge. An ionic bond, also known as an electrovalent. Are Ionic Bonds Lustrous.
From slideplayer.com
Introduction to Dentistry and Biomaterials ppt download Are Ionic Bonds Lustrous Ionic compounds are poor conductors in the solid state. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic compounds are hard and brittle. Ionic compounds have high melting points. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between. Are Ionic Bonds Lustrous.
From www.vedantu.com
Exploring the Key Difference between ionic covalent and metallic bonds Are Ionic Bonds Lustrous Most ionic solids, however, dissolve. Ionic compounds have high melting points. Ionic compounds are hard and brittle. The reason is—the strength of ionic bonds prevents ions from moving freely in the solid state. Solutions of ionic compounds and melted ionic compounds conduct. What is an ionic bond? The ions may either be monatomic or polyatomic. Ionic bond, type of linkage. Are Ionic Bonds Lustrous.