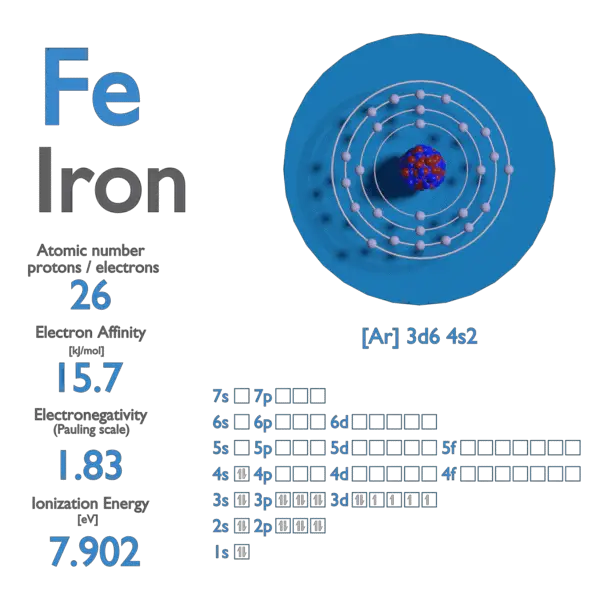
Iron Electron Affinity . The amount of energy required to remove the. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. use the trends in electron affinities going down a column for elements in the same group. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion.
from www.nuclear-power.com
125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. The amount of energy required to remove the. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. use the trends in electron affinities going down a column for elements in the same group. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous.
Iron Electron Affinity Electronegativity Ionization Energy of
Iron Electron Affinity The amount of energy required to remove the. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. The amount of energy required to remove the. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. use the trends in electron affinities going down a column for elements in the same group. to recognize the inverse relationship of ionization energies and electron affinities.
From sciencenotes.org
Electron Affinity Trend and Definition Iron Electron Affinity the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. to recognize the inverse relationship of ionization energies and electron affinities. 125 rows the electron affinity is defined as the amount of energy released when an electron is. Iron Electron Affinity.
From www.nuclear-power.com
Iron Electron Affinity Electronegativity Ionization Energy of Iron Electron Affinity use the trends in electron affinities going down a column for elements in the same group. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous. Iron Electron Affinity.
From mydigitalkemistry.com
Electron Affinity Trends Chemistry Digital Kemistry Best Online Iron Electron Affinity electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. to recognize the inverse relationship of ionization energies and electron affinities. The amount. Iron Electron Affinity.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Iron Electron Affinity use the trends in electron affinities going down a column for elements in the same group. to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. the electron affinity of an element is the energy. Iron Electron Affinity.
From dxopgqpsw.blob.core.windows.net
Iron Electron Density at Arlyne Henry blog Iron Electron Affinity to recognize the inverse relationship of ionization energies and electron affinities. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the. Iron Electron Affinity.
From chem.libretexts.org
1.1.2.4 Electron Affinity Chemistry LibreTexts Iron Electron Affinity the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. electron affinity is defined. Iron Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Iron Electron Affinity the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is the amount. Iron Electron Affinity.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID855242 Iron Electron Affinity electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. use the trends in electron affinities going down a column for elements in the same group. 125 rows the electron affinity is defined as the amount of energy released when an electron is. Iron Electron Affinity.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Electron Affinity to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra. Iron Electron Affinity.
From www.jove.com
Periodic Properties of Elements Electron Affinity JoVE Book Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. use the trends in electron affinities going down a column for elements in the same group. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the. Iron Electron Affinity.
From eduinput.com
Electron Affinity, definition, examples, significance, factors Iron Electron Affinity The amount of energy required to remove the. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity. Iron Electron Affinity.
From www.youtube.com
Electron Affinity Part2 Chemistry General Concepts YouTube Iron Electron Affinity The amount of energy required to remove the. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. . Iron Electron Affinity.
From pediabay.com
Electron Affinity Chart of Elements (With Periodic Table) Pediabay Iron Electron Affinity the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. to recognize the inverse relationship of ionization energies and electron affinities. 125 rows the electron affinity is defined as the amount of energy released when an electron is. Iron Electron Affinity.
From www.slideserve.com
PPT Chapter 4 The Periodic Table PowerPoint Presentation, free Iron Electron Affinity The amount of energy required to remove the. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. . Iron Electron Affinity.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Iron Electron Affinity the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. to recognize the inverse relationship of ionization energies and electron affinities. use the trends in electron affinities going down a column for elements in the same group. . Iron Electron Affinity.
From www.breakingatom.com
Electron Affinity of The Elements Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. to recognize the inverse relationship of ionization energies and electron. Iron Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. use the trends in electron affinities going down a column for elements in the same group. to recognize the inverse relationship of ionization energies and electron affinities. The amount of energy required to remove. Iron Electron Affinity.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. to recognize the inverse relationship of ionization energies and electron. Iron Electron Affinity.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. use the trends in electron affinities going down a column for elements in the. Iron Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Iron Electron Affinity to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. 125. Iron Electron Affinity.
From dxopgqpsw.blob.core.windows.net
Iron Electron Density at Arlyne Henry blog Iron Electron Affinity the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. use the trends in. Iron Electron Affinity.
From www.nuclear-power.com
Electron Affinity Iron Electron Affinity The amount of energy required to remove the. use the trends in electron affinities going down a column for elements in the same group. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. the electron affinity of an element is the energy given. Iron Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Iron Electron Affinity electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. the electron affinity of an element is the energy given off when a. Iron Electron Affinity.
From www.slideserve.com
PPT Periodic Properties of Elements PowerPoint Presentation, free Iron Electron Affinity The amount of energy required to remove the. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. . Iron Electron Affinity.
From www.breakingatom.com
Electron Affinity of The Elements Iron Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is the amount of energy change (δe) that occurs when an electron. Iron Electron Affinity.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. The amount of energy required to remove the. to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is the amount of energy change (δe) that occurs when an electron is. Iron Electron Affinity.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron Electron Affinity use the trends in electron affinities going down a column for elements in the same group. to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 125 rows the electron affinity is defined as the. Iron Electron Affinity.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. use the trends in electron affinities going down a column for elements in the same group. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Iron Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Iron Electron Affinity electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. The amount of energy required to remove the. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity. Iron Electron Affinity.
From adriandingleschemistrypages.com
Periodicity A couple more things Adrian Dingle's Chemistry Pages Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. The amount of energy required to remove the. to recognize. Iron Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Iron Electron Affinity the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. The amount of energy required to remove the. use the trends in electron affinities going down a column for elements in the same group. electron affinity is the. Iron Electron Affinity.
From dxopgqpsw.blob.core.windows.net
Iron Electron Density at Arlyne Henry blog Iron Electron Affinity to recognize the inverse relationship of ionization energies and electron affinities. the electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. The amount of energy required to remove the. electron affinity is defined as the change in energy. Iron Electron Affinity.
From mungfali.com
Electronegativity And Electron Affinity Iron Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. to recognize the inverse relationship of ionization energies and electron affinities. The amount of energy required to remove the. electron affinity is the amount of energy change (δe) that occurs when an electron is. Iron Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Iron Electron Affinity to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. The amount of energy required to remove the. use the trends in electron affinities going down a column for elements in the. Iron Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Iron Electron Affinity electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. to recognize the inverse relationship of ionization energies and electron affinities. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 125. Iron Electron Affinity.