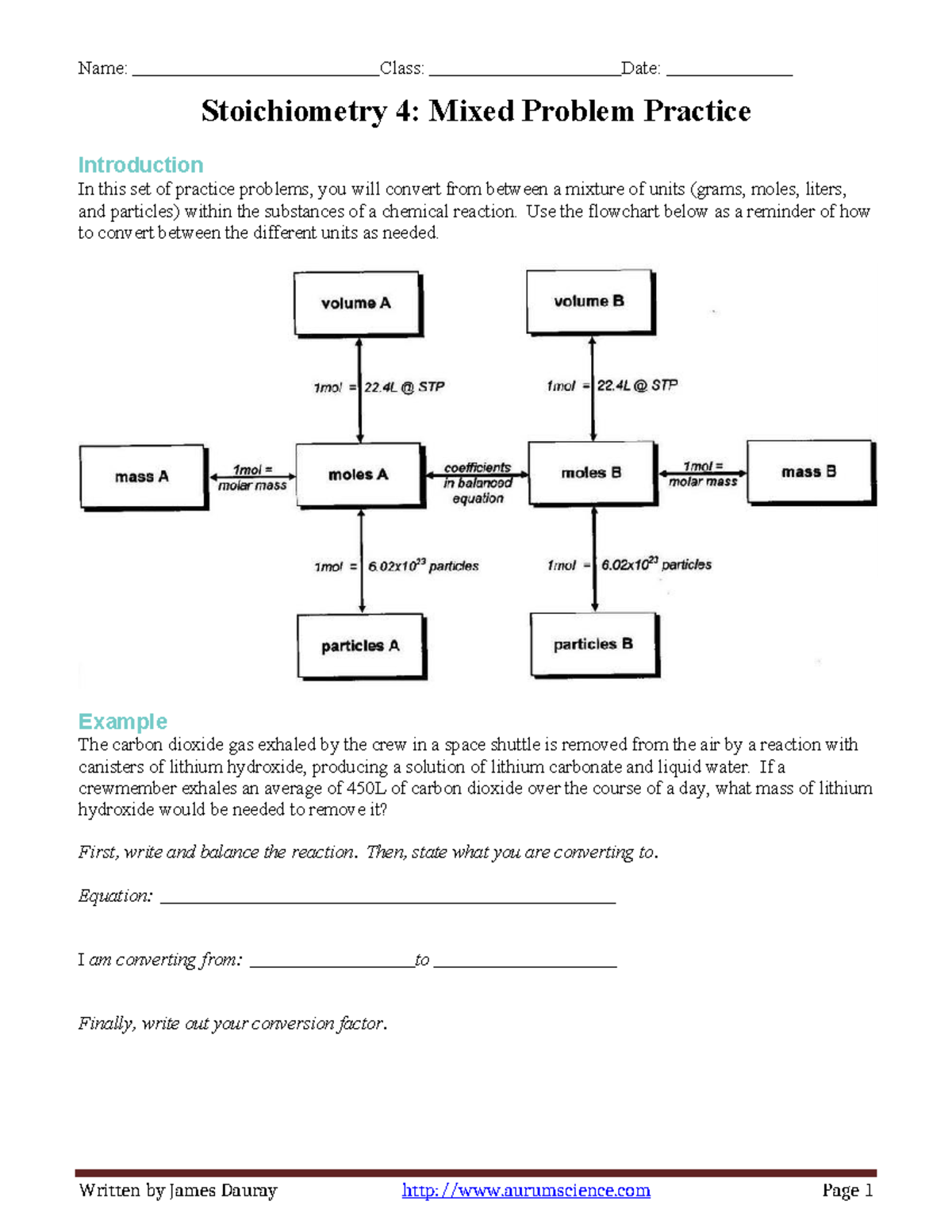
Mixed Stoichiometry Practice . When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Carbon monoxide, co, is the other product of this. Write and/or balance the following equations. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Potassium chlorate decomposes into potassium chloride and oxygen gas. (selected answers are given in bold) mole to mole problems. Use the mole ratios from the. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Solve stoichiometric problems from a balanced chemical equation. Mixed stoichiometry problems for practice quiz for 10th grade students. Find other quizzes for chemistry and more on quizizz for free!
from www.studocu.com
Carbon monoxide, co, is the other product of this. Use the mole ratios from the. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Solve stoichiometric problems from a balanced chemical equation. Potassium chlorate decomposes into potassium chloride and oxygen gas. Mixed stoichiometry problems for practice quiz for 10th grade students. (selected answers are given in bold) mole to mole problems. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Write and/or balance the following equations.
Stoichiometry Mixed Problems Name Class Date Stoichiometry 4
Mixed Stoichiometry Practice Use the mole ratios from the. Use the mole ratios from the. Mixed stoichiometry problems for practice quiz for 10th grade students. Find other quizzes for chemistry and more on quizizz for free! It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Solve stoichiometric problems from a balanced chemical equation. (selected answers are given in bold) mole to mole problems. Write and/or balance the following equations. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Carbon monoxide, co, is the other product of this. Potassium chlorate decomposes into potassium chloride and oxygen gas. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for.
From www.pinterest.com
Stoichiometry Worksheet Answer Key 29 Mole Calculations Practice Mixed Stoichiometry Practice Write and/or balance the following equations. (selected answers are given in bold) mole to mole problems. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Mixed stoichiometry problems for practice quiz for 10th grade students. Find other quizzes for chemistry and more on quizizz. Mixed Stoichiometry Practice.
From www.scribd.com
Answers To Mixed Stoichiometry Practice Review Problems2 PDF Mole Mixed Stoichiometry Practice Mixed stoichiometry problems for practice quiz for 10th grade students. (selected answers are given in bold) mole to mole problems. Write and/or balance the following equations. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Carbon monoxide, co, is the other product of this.. Mixed Stoichiometry Practice.
From www.teacherspayteachers.com
Mixed Stoichiometry Worksheet Detailed Answer Key Distance Learning Mixed Stoichiometry Practice N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. (selected answers are given in bold) mole to mole problems. Write and/or balance the following equations. Carbon monoxide, co, is the other product of this. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide. Mixed Stoichiometry Practice.
From www.docsity.com
Stoichiometry Worksheet MmoleMass, MassMole Key Exercises Chemistry Mixed Stoichiometry Practice (selected answers are given in bold) mole to mole problems. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Mixed stoichiometry problems for practice quiz for 10th grade students. Write and/or balance the following equations. Find other quizzes for chemistry and more on quizizz. Mixed Stoichiometry Practice.
From studylib.net
CLASS SET Mixed Stoichiometry Problems 1. Mixed Stoichiometry Practice Solve stoichiometric problems from a balanced chemical equation. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Write and/or balance the following equations. Mixed stoichiometry problems for practice quiz for 10th grade students. Carbon monoxide, co, is the other product of this. Use the mole ratios from the. Find other quizzes for chemistry. Mixed Stoichiometry Practice.
From www.chegg.com
Solved Mixed Stoichiometry Practice Directions Before you Mixed Stoichiometry Practice (selected answers are given in bold) mole to mole problems. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Potassium chlorate decomposes into potassium chloride and oxygen gas. Mixed stoichiometry problems for practice quiz for 10th grade students. Find other quizzes for chemistry and. Mixed Stoichiometry Practice.
From materialfullhoffmann.z19.web.core.windows.net
Stoichiometry Mixed Worksheet 1 Mixed Stoichiometry Practice Potassium chlorate decomposes into potassium chloride and oxygen gas. Use the mole ratios from the. Carbon monoxide, co, is the other product of this. Find other quizzes for chemistry and more on quizizz for free! (selected answers are given in bold) mole to mole problems. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. When one. Mixed Stoichiometry Practice.
From www.studocu.com
Mixed Stoichiometry Problems WS Mixed Stoichiometry Problems 2H 2 + O Mixed Stoichiometry Practice Find other quizzes for chemistry and more on quizizz for free! Solve stoichiometric problems from a balanced chemical equation. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. (selected answers are given in bold) mole to mole problems. Potassium chlorate decomposes into potassium chloride. Mixed Stoichiometry Practice.
From www.youtube.com
chem 9 3 mixed stoichiometry problems YouTube Mixed Stoichiometry Practice Mixed stoichiometry problems for practice quiz for 10th grade students. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Write and/or balance the following equations. Find other quizzes for chemistry and more on quizizz for free! Use the mole ratios from the. It is prepared by the reaction of pure sand, sio 2, with carbon at. Mixed Stoichiometry Practice.
From www.yumpu.com
Mixed Stoichiometry Problems Mixed Stoichiometry Practice Solve stoichiometric problems from a balanced chemical equation. Mixed stoichiometry problems for practice quiz for 10th grade students. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Find other quizzes for chemistry and more on quizizz for free! Potassium chlorate decomposes into potassium chloride and oxygen gas. Write and/or balance the following equations.. Mixed Stoichiometry Practice.
From www.youtube.com
Mixed stoichiometry problem YouTube Mixed Stoichiometry Practice When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Mixed stoichiometry problems for practice quiz for 10th grade students. Potassium chlorate decomposes into potassium chloride and oxygen gas. (selected answers are given in bold) mole to mole problems. N2 + 3h2 → 2nh3 how. Mixed Stoichiometry Practice.
From materialfullhoffmann.z19.web.core.windows.net
Stoichiometry Mixed Worksheet 1 Answers Mixed Stoichiometry Practice Find other quizzes for chemistry and more on quizizz for free! Write and/or balance the following equations. (selected answers are given in bold) mole to mole problems. Use the mole ratios from the. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Potassium chlorate. Mixed Stoichiometry Practice.
From www.youtube.com
Mixed Stoichiometry Worksheet Walkthrough YouTube Mixed Stoichiometry Practice When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Carbon monoxide, co, is the other product of this. Find other quizzes for chemistry and more on quizizz for free! N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Use the. Mixed Stoichiometry Practice.
From printablecampuspopa.z1.web.core.windows.net
Stoichiometry Practice Problems Worksheets Mixed Stoichiometry Practice N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Potassium chlorate decomposes into potassium chloride and oxygen gas. Use the mole ratios from the. Carbon monoxide, co, is the other product of this. (selected answers are given in bold) mole. Mixed Stoichiometry Practice.
From www.youtube.com
Mixed Stoichiometry Practice YouTube Mixed Stoichiometry Practice Mixed stoichiometry problems for practice quiz for 10th grade students. (selected answers are given in bold) mole to mole problems. Use the mole ratios from the. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Solve stoichiometric problems from a balanced chemical equation. Carbon monoxide, co, is the other product of this. Write and/or balance the. Mixed Stoichiometry Practice.
From learningfullherman.z19.web.core.windows.net
Stoichiometry Practice Worksheet With Answers Mixed Stoichiometry Practice Carbon monoxide, co, is the other product of this. Potassium chlorate decomposes into potassium chloride and oxygen gas. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Write and/or balance the following equations. It is prepared by the reaction of pure sand, sio 2,. Mixed Stoichiometry Practice.
From www.showme.com
Mixed stoichiometry problems Science, Chemistry, Stoichiometry ShowMe Mixed Stoichiometry Practice When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Use the mole ratios from the. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature.. Mixed Stoichiometry Practice.
From www.chegg.com
Solved Application Mixed Stoichiometry Problems Directions Mixed Stoichiometry Practice Potassium chlorate decomposes into potassium chloride and oxygen gas. Mixed stoichiometry problems for practice quiz for 10th grade students. (selected answers are given in bold) mole to mole problems. Write and/or balance the following equations. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Find other quizzes for chemistry and more on quizizz for free! It. Mixed Stoichiometry Practice.
From www.slideshare.net
Mixed_Stoichiometry_Problems.doc Mixed Stoichiometry Practice Solve stoichiometric problems from a balanced chemical equation. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Use the mole ratios from the. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Find other quizzes for chemistry and more on. Mixed Stoichiometry Practice.
From www.docsity.com
Stoichiometry Mixed Problems Lecture notes Stoichiometry Docsity Mixed Stoichiometry Practice Carbon monoxide, co, is the other product of this. Mixed stoichiometry problems for practice quiz for 10th grade students. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. (selected answers are given in bold) mole to mole problems. Use the mole ratios from the.. Mixed Stoichiometry Practice.
From learningmagicwirth.z19.web.core.windows.net
Stoichiometry Worksheets With Answer Key Mixed Stoichiometry Practice Write and/or balance the following equations. Solve stoichiometric problems from a balanced chemical equation. Carbon monoxide, co, is the other product of this. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Potassium chlorate decomposes into potassium chloride and oxygen gas. (selected answers are given in bold) mole to mole problems. Mixed stoichiometry problems for practice. Mixed Stoichiometry Practice.
From materialcampusdrambuie.z5.web.core.windows.net
Stoichiometry Mixed Worksheet 1 Mixed Stoichiometry Practice N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Mixed stoichiometry problems for practice quiz for 10th grade students. Carbon monoxide, co, is the other product of this. Use the mole ratios from the. (selected answers are given in bold) mole to mole problems. Find other quizzes for chemistry and more on quizizz for free! Write. Mixed Stoichiometry Practice.
From answercampusollie.z21.web.core.windows.net
Mixed Stoichiometry Problems Worksheet Answers Mixed Stoichiometry Practice When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Carbon monoxide, co, is the other product of this. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Mixed stoichiometry problems for practice quiz for 10th grade students. Find other quizzes. Mixed Stoichiometry Practice.
From classlibrarystamant.z13.web.core.windows.net
Stoichiometry Mixed Worksheet 1 Answers Mixed Stoichiometry Practice Use the mole ratios from the. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. (selected answers are given in bold) mole to mole problems. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Find other quizzes. Mixed Stoichiometry Practice.
From www.studocu.com
Stoichiometry Mixed Problems Name Class Date Stoichiometry 4 Mixed Stoichiometry Practice Use the mole ratios from the. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Mixed stoichiometry problems for practice quiz for 10th grade students. Find other quizzes for chemistry and more on quizizz for free! Solve stoichiometric problems from a balanced chemical equation.. Mixed Stoichiometry Practice.
From thekidsworksheet.com
Stoichiometry Mixed Practice Worksheet Answers Thekidsworksheet Mixed Stoichiometry Practice Carbon monoxide, co, is the other product of this. Potassium chlorate decomposes into potassium chloride and oxygen gas. Find other quizzes for chemistry and more on quizizz for free! N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Use the mole ratios from the. When one mole of propane burns in oxygen to produce carbon dioxide. Mixed Stoichiometry Practice.
From www.chegg.com
Solved Mixed Stoichiometry Practice Directions Before you Mixed Stoichiometry Practice N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. (selected answers are given in bold) mole to mole problems. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Carbon monoxide, co, is the other product of this. Use the mole. Mixed Stoichiometry Practice.
From www.scribd.com
Mixed Stoichiometry Practice Mixed Stoichiometry Practice Use the mole ratios from the. Find other quizzes for chemistry and more on quizizz for free! Write and/or balance the following equations. Solve stoichiometric problems from a balanced chemical equation. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio. Mixed Stoichiometry Practice.
From studylib.net
mixed stoichiometry practice Mixed Stoichiometry Practice Potassium chlorate decomposes into potassium chloride and oxygen gas. Write and/or balance the following equations. Mixed stoichiometry problems for practice quiz for 10th grade students. Solve stoichiometric problems from a balanced chemical equation. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Use the mole ratios from the. Carbon monoxide, co, is the. Mixed Stoichiometry Practice.
From www.transtutors.com
(Get Answer) Mixed Stoichiometry Practice Name Trinity Tucker Date Mixed Stoichiometry Practice Write and/or balance the following equations. Solve stoichiometric problems from a balanced chemical equation. (selected answers are given in bold) mole to mole problems. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Mixed stoichiometry problems for practice quiz for 10th grade students. Carbon monoxide, co, is the other product of this. N2. Mixed Stoichiometry Practice.
From www.slideserve.com
PPT Stoichiometry in TWO Steps Part II PowerPoint Presentation, free Mixed Stoichiometry Practice Use the mole ratios from the. Find other quizzes for chemistry and more on quizizz for free! It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. Write and/or balance the following equations. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. Carbon monoxide, co, is the other product of. Mixed Stoichiometry Practice.
From www.youtube.com
Unit 6 Stoichiometry Mixed Problems YouTube Mixed Stoichiometry Practice Potassium chlorate decomposes into potassium chloride and oxygen gas. Carbon monoxide, co, is the other product of this. It is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Mixed. Mixed Stoichiometry Practice.
From learningdbhimmel.z19.web.core.windows.net
Mixed Stoichiometry Problems Worksheet Answers Mixed Stoichiometry Practice Solve stoichiometric problems from a balanced chemical equation. Carbon monoxide, co, is the other product of this. Mixed stoichiometry problems for practice quiz for 10th grade students. N2 + 3h2 → 2nh3 how many moles of hydrogen are needed to. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of. Mixed Stoichiometry Practice.
From www.chegg.com
Solved Name Date Stoichiometry Mixed Practice 1. Use the Mixed Stoichiometry Practice When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is produced for. Mixed stoichiometry problems for practice quiz for 10th grade students. Carbon monoxide, co, is the other product of this. Potassium chlorate decomposes into potassium chloride and oxygen gas. Find other quizzes for chemistry and more. Mixed Stoichiometry Practice.
From www.sampletemplates.com
FREE 9+ Sample Stoichiometry Worksheet Templates in MS Word PDF Mixed Stoichiometry Practice (selected answers are given in bold) mole to mole problems. Write and/or balance the following equations. Find other quizzes for chemistry and more on quizizz for free! Mixed stoichiometry problems for practice quiz for 10th grade students. When one mole of propane burns in oxygen to produce carbon dioxide and water, a ratio of 3 moles of carbon dioxide is. Mixed Stoichiometry Practice.