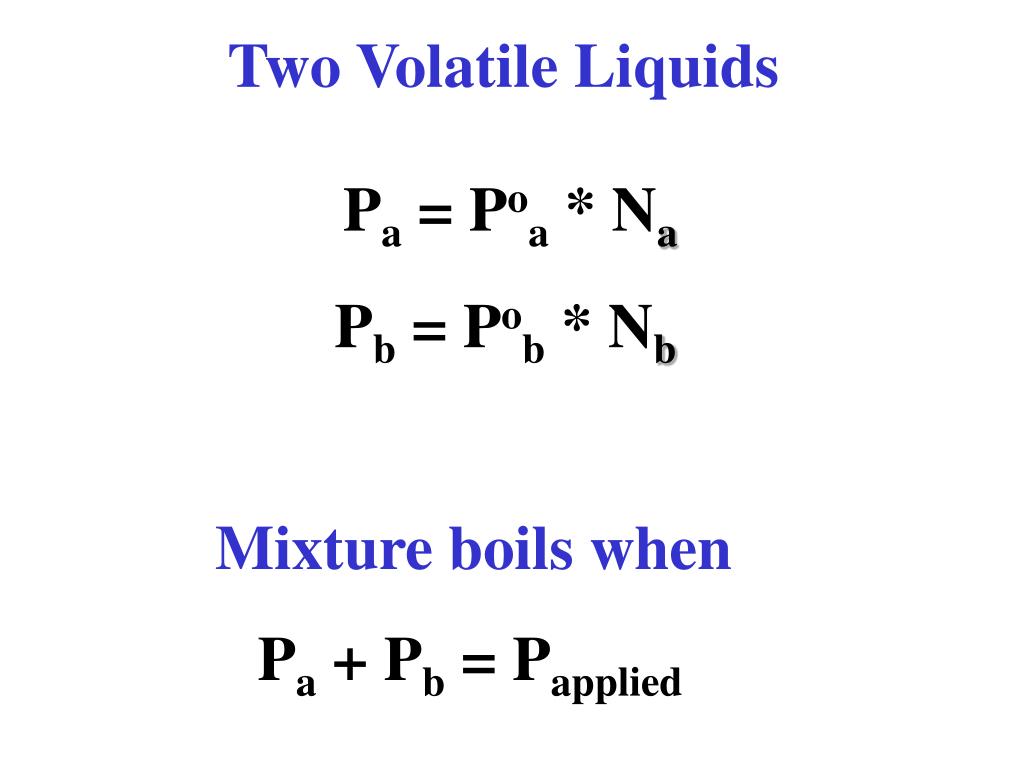
Volatile Vs Non Volatile Liquids . Both elements have high vapor pressures and readily release particles into. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile substances are characterized by. When the vapor pressure equals. Nonvolatile liquids have low vapor pressures. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure.
from www.slideserve.com
Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. When the vapor pressure equals. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Both elements have high vapor pressures and readily release particles into. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;
PPT Boiling Points Distillations PowerPoint Presentation, free
Volatile Vs Non Volatile Liquids Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. When the vapor pressure equals. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. Both elements have high vapor pressures and readily release particles into. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable.
From www.differencebetween.com
Difference Between Volatile and Nonvolatile Acids Compare the Volatile Vs Non Volatile Liquids When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Both elements have. Volatile Vs Non Volatile Liquids.
From chemistry.stackexchange.com
physical chemistry What causes the lowering of vapour pressure in Volatile Vs Non Volatile Liquids Volatile substances are characterized by. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; When the vapor pressure equals. Both elements have high vapor pressures and readily release particles into. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Nonvolatile liquids have low vapor. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Memory systems PowerPoint Presentation, free download ID6237422 Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid,. Volatile Vs Non Volatile Liquids.
From slideplayer.com
Liquids and Solids Changes of State. ppt video online download Volatile Vs Non Volatile Liquids Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Both elements have high vapor pressures and readily release particles into. Nonvolatile liquids have low vapor pressures. Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid,. Volatile Vs Non Volatile Liquids.
From slidetodoc.com
Volatile Substances Volatile Substances Commonly referred to as Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from. Volatile Vs Non Volatile Liquids.
From www.youtube.com
4.8 Volatile vs Non volatile Memory YouTube Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; When the vapor pressure equals. Nonvolatile liquids have low vapor pressures. Both elements have high vapor pressures and readily release particles into. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low vapor. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Liquids & Solids PowerPoint Presentation, free download ID6068076 Volatile Vs Non Volatile Liquids Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. Nonvolatile liquids have low vapor pressures. When the vapor pressure equals. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile liquids are liquids. Volatile Vs Non Volatile Liquids.
From www.askdifference.com
Volatile vs. Nonvolatile — What’s the Difference? Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile substances are characterized by. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Mercury (hg) and bromine. Volatile Vs Non Volatile Liquids.
From www.youtube.com
State Raoult's law for volatile solutes and for nonvolatile solute Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT A.P. Chemistry PowerPoint Presentation, free download ID1875178 Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. When the vapor pressure equals. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate. Volatile Vs Non Volatile Liquids.
From slideplayer.com
Electrolytes. Ionic compounds Break down into positive and negative Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low vapor pressures. When the vapor. Volatile Vs Non Volatile Liquids.
From sciencenotes.org
Volatility Volatile Definition in Chemistry Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; When the vapor pressure equals. Both elements have high vapor pressures and readily release particles into. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. Mercury (hg) and bromine. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Boiling Points Distillations PowerPoint Presentation, free Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Volatile substances are characterized by. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and. Volatile Vs Non Volatile Liquids.
From www.researchgate.net
Interfacial concentration of volatile and non volatile liquids as a Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. Nonvolatile liquids have low vapor pressures. When the vapor pressure equals. Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile liquids are liquids with high vapor pressures, which tend. Volatile Vs Non Volatile Liquids.
From www.youtube.com
Separation of Volatile liquid solid mixture by Distillation; BSc Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile substances are characterized by. Nonvolatile liquids have. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Analysis for Total Solids PowerPoint Presentation, free download Volatile Vs Non Volatile Liquids Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Nonvolatile liquids have low vapor pressures. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; When the. Volatile Vs Non Volatile Liquids.
From www.youtube.com
Volatile vs NonVolatile Memory Simply Explained YouTube Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. When the vapor pressure equals. Volatile substances are characterized by. Nonvolatile liquids have low vapor pressures. Both elements have high vapor pressures and readily release particles. Volatile Vs Non Volatile Liquids.
From www.youtube.com
Type detection for liquid mixture Volatile liquidsolid, VolatileNon Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low vapor pressures. When the vapor pressure equals. Nonvolatile liquids have low vapor. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Phase Changes and Heat Calculations PowerPoint Presentation, free Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; When the vapor pressure equals. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Nonvolatile liquids have low vapor pressures. Both elements have high vapor pressures and readily release particles into. Volatile liquids are. Volatile Vs Non Volatile Liquids.
From www.diffzy.com
Volatile vs. Non Volatile Storage What's The Difference (With Table) Volatile Vs Non Volatile Liquids Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. Both elements have high vapor pressures and readily release particles into. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. When the vapor pressure equals. Volatile liquids are liquids with high vapor pressures, which tend. Volatile Vs Non Volatile Liquids.
From pediaa.com
Difference Between Volatile and Nonvolatile Substances Definition Volatile Vs Non Volatile Liquids Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Volatile substances are characterized by. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an. Volatile Vs Non Volatile Liquids.
From worldtechjournal.com
Volatile And Non Volatile Memory Best 6 Differences World Tech Journal Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Volatile substances are characterized by. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Volatile liquids are liquids with high vapor pressures, which tend. Volatile Vs Non Volatile Liquids.
From byjus.com
On adding a non volatile solute to a volatile solvent Volatile Vs Non Volatile Liquids Nonvolatile liquids have low vapor pressures. When the vapor pressure equals. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Both elements have high vapor pressures and readily release particles. Volatile Vs Non Volatile Liquids.
From www.slideshare.net
Chapter 11 Properties of Solutions Volatile Vs Non Volatile Liquids Volatile substances are characterized by. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. When the vapor pressure equals. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open. Volatile Vs Non Volatile Liquids.
From www.slideshare.net
Chapter 12.3 Changes of State Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. When the vapor pressure equals. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure.. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Chapter 10 Properties of Solutions PowerPoint Presentation, free Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; When the vapor pressure equals. Both elements have high vapor pressures and readily release particles into. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile substances are characterized by. Volatile substances easily vaporize at. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Volatile Vs Non Volatile Liquids When the vapor pressure equals. Nonvolatile liquids have low vapor pressures. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Both elements have high vapor pressures and readily release. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT 4.5 Physical Properties of Covalent Molecules PowerPoint Volatile Vs Non Volatile Liquids Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. When the vapor pressure equals. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Both elements have. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Liquids & Solids PowerPoint Presentation, free download ID6068076 Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile substances are characterized by. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Both elements have high vapor. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID59727 Volatile Vs Non Volatile Liquids Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Both elements have high vapor pressures and readily release particles into. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Storage PowerPoint Presentation, free download ID7100032 Volatile Vs Non Volatile Liquids Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Nonvolatile liquids have low vapor pressures. Volatile substances are characterized by. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an. Volatile Vs Non Volatile Liquids.
From www.differencebetween.com
Difference Between Volatile and Nonvolatile Compare the Difference Volatile Vs Non Volatile Liquids Volatile substances are characterized by. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Both elements have high vapor pressures and readily release particles into. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Nonvolatile liquids have low vapor pressures. Volatile substances easily vaporize. Volatile Vs Non Volatile Liquids.
From dreamscomputerlearning.blogspot.com
Difference between Volatile memory and Non Volatile Memory Volatile Vs Non Volatile Liquids Volatile substances are characterized by. When the vapor pressure equals. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Nonvolatile liquids have low vapor pressures. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do. Volatile Vs Non Volatile Liquids.
From pediaa.com
Difference Between Volatile and Nonvolatile Substances Definition Volatile Vs Non Volatile Liquids Both elements have high vapor pressures and readily release particles into. Volatile substances easily vaporize at low temperatures, while nonvolatile substances do not readily evaporate and remain stable. Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend. Volatile Vs Non Volatile Liquids.
From www.slideserve.com
PPT Liquids & Solids PowerPoint Presentation, free download ID6068076 Volatile Vs Non Volatile Liquids Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Mercury (hg) and bromine (br) are volatile elements that are liquids at ordinary temperature and pressure. Nonvolatile liquids have low vapor pressures. Nonvolatile liquids have low vapor pressures. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid,. Volatile Vs Non Volatile Liquids.