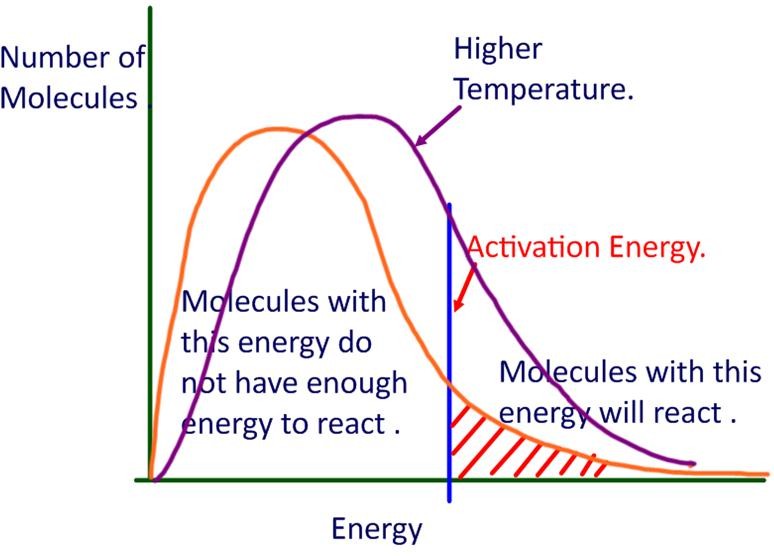
Collision Theory Effect Of Temperature On Reaction Rate . This page describes and explains the way that changing the temperature affects the rate of a reaction. In this section, we will use the collision model to analyze this relationship between. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. There are several factors that can affect the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Diagram showing the increased kinetic energy that particles have at higher temperatures. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. It assumes that you are already familiar with basic ideas about the collision theory, and. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The arrhenius equation describes the relation between a reaction’s. The rate of the reaction depends.
from blogs.glowscotland.org.uk
Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. It assumes that you are already familiar with basic ideas about the collision theory, and. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The rate of the reaction depends. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Diagram showing the increased kinetic energy that particles have at higher temperatures. There are several factors that can affect the rate of a reaction. The arrhenius equation describes the relation between a reaction’s. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another.
Collision Theory and Rate of Reaction Higher Chemistry Unit 1
Collision Theory Effect Of Temperature On Reaction Rate The rate of the reaction depends. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Diagram showing the increased kinetic energy that particles have at higher temperatures. There are several factors that can affect the rate of a reaction. This page describes and explains the way that changing the temperature affects the rate of a reaction. The arrhenius equation describes the relation between a reaction’s. In this section, we will use the collision model to analyze this relationship between. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of the reaction depends. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates It assumes that you are already familiar with basic ideas about the collision theory, and.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Collision Theory Effect Of Temperature On Reaction Rate This page describes and explains the way that changing the temperature affects the rate of a reaction. There are several factors that can affect the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory, and. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. Use the. Collision Theory Effect Of Temperature On Reaction Rate.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation Collision Theory Effect Of Temperature On Reaction Rate Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. It assumes that you are already familiar with basic ideas about the collision theory, and. Diagram showing the increased kinetic energy that particles have at higher temperatures. The arrhenius equation describes the relation between a reaction’s. The rate of the reaction depends.. Collision Theory Effect Of Temperature On Reaction Rate.
From thescienceandmathszone.com
Simple Collision Theory The Science and Maths Zone Collision Theory Effect Of Temperature On Reaction Rate Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. In this section, we will use the collision model to analyze this relationship between. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Diagram showing the increased kinetic energy. Collision Theory Effect Of Temperature On Reaction Rate.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Theory Effect Of Temperature On Reaction Rate In this section, we will use the collision model to analyze this relationship between. The arrhenius equation describes the relation between a reaction’s. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. Collision Theory Effect Of Temperature On Reaction Rate.
From www.sciencelearn.org.nz
Rate of reaction and temperature — Science Learning Hub Collision Theory Effect Of Temperature On Reaction Rate The collision model explains why chemical reactions often occur more rapidly at higher temperatures. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The arrhenius equation describes the relation between a reaction’s. In this section, we will use the collision model to analyze this relationship between. Diagram showing the increased kinetic. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Chapter 14 Section 5 The Effect of Temperature on Reaction Rate YouTube Collision Theory Effect Of Temperature On Reaction Rate The rate of the reaction depends. There are several factors that can affect the rate of a reaction. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory provides a simple but effective explanation for the. Collision Theory Effect Of Temperature On Reaction Rate.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Collision Theory Effect Of Temperature On Reaction Rate There are several factors that can affect the rate of a reaction. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The rate of the reaction depends. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory provides a simple. Collision Theory Effect Of Temperature On Reaction Rate.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Collision Theory Effect Of Temperature On Reaction Rate Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The arrhenius equation describes the relation between a reaction’s. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. There are several factors that can affect the rate of a reaction. It assumes that. Collision Theory Effect Of Temperature On Reaction Rate.
From pablogroeaton.blogspot.com
Collision Theory of Reaction Rates PablogroEaton Collision Theory Effect Of Temperature On Reaction Rate For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Diagram showing the increased kinetic energy that particles have at higher temperatures. There are several factors that can affect the rate of a reaction. Collision theory states that for a chemical reaction to occur, the reacting particles must. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Effect of Temperature on Rates of a Reversible Reaction at Equilibrium Collision Theory Effect Of Temperature On Reaction Rate In this section, we will use the collision model to analyze this relationship between. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. This page describes and explains the way that changing the temperature affects the rate of a reaction. There are several factors that can affect the rate of a reaction. Collision theory states. Collision Theory Effect Of Temperature On Reaction Rate.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID9507407 Collision Theory Effect Of Temperature On Reaction Rate The rate of the reaction depends. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Diagram showing the increased kinetic energy that particles have at higher temperatures. Collision theory provides a simple but. Collision Theory Effect Of Temperature On Reaction Rate.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Theory Effect Of Temperature On Reaction Rate Diagram showing the increased kinetic energy that particles have at higher temperatures. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates There are several factors that can affect the rate of a. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Collision Theory Effect Of Temperature On Reaction Rate Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. This page describes and explains. Collision Theory Effect Of Temperature On Reaction Rate.
From app.jove.com
Effect of Temperature on Reaction Rate, Arrhenius Equation and Collision Theory Effect Of Temperature On Reaction Rate For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. This page describes and explains the way that changing the temperature affects the rate of a reaction. Collision theory provides a simple but effective. Collision Theory Effect Of Temperature On Reaction Rate.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Collision Theory Effect Of Temperature On Reaction Rate Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. There are several factors that can affect the rate of a reaction. For example, the reaction rates of many reactions that occur at room. Collision Theory Effect Of Temperature On Reaction Rate.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Collision Theory Effect Of Temperature On Reaction Rate The rate of the reaction depends. In this section, we will use the collision model to analyze this relationship between. There are several factors that can affect the rate of a reaction. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The collision model explains why chemical reactions often occur more. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Factors Affecting Rate of Reaction + Collision Theory GCSE Science Collision Theory Effect Of Temperature On Reaction Rate For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. There are several factors that can affect the rate of a reaction. In this section, we will use the collision model to analyze this relationship between. Collision theory provides a simple but effective explanation for the effect of. Collision Theory Effect Of Temperature On Reaction Rate.
From www.shalom-education.com
Effect of Temperature on the Rate of Reaction GCSE Chemistry Revision Collision Theory Effect Of Temperature On Reaction Rate The arrhenius equation describes the relation between a reaction’s. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. In this section, we will use the collision model to analyze this relationship between. Diagram showing the increased kinetic energy that particles have at higher temperatures. Collision theory provides a simple but effective. Collision Theory Effect Of Temperature On Reaction Rate.
From integratedsciencegeneral11.weebly.com
Collision Theory Explains Chemical Reactions Integrated Science 11 Collision Theory Effect Of Temperature On Reaction Rate For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. This page describes and explains the way that changing the temperature affects the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Diagram showing the. Collision Theory Effect Of Temperature On Reaction Rate.
From chem.libretexts.org
14.5 The Effect of Temperature on Reaction Rate Chemistry LibreTexts Collision Theory Effect Of Temperature On Reaction Rate For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates There are several factors that can affect the rate of a reaction. It assumes that you are already familiar. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Collision Theory. Effect of temperature and concentration on rate Collision Theory Effect Of Temperature On Reaction Rate There are several factors that can affect the rate of a reaction. Diagram showing the increased kinetic energy that particles have at higher temperatures. In this section, we will use the collision model to analyze this relationship between. This page describes and explains the way that changing the temperature affects the rate of a reaction. It assumes that you are. Collision Theory Effect Of Temperature On Reaction Rate.
From app.jove.com
Effect of Temperature on Reaction Rate, Arrhenius Equation and Collision Theory Effect Of Temperature On Reaction Rate Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. In this section, we will use the collision model to analyze this relationship between. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. There are several factors that can affect the rate of a reaction. Use the. Collision Theory Effect Of Temperature On Reaction Rate.
From www.studypool.com
SOLUTION Effect of temperature on reaction rate collision theory Collision Theory Effect Of Temperature On Reaction Rate Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. It assumes that you are already familiar with basic ideas about the collision theory, and. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The collision model explains why chemical reactions often. Collision Theory Effect Of Temperature On Reaction Rate.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Theory Effect Of Temperature On Reaction Rate There are several factors that can affect the rate of a reaction. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. This page describes and explains the way that changing the temperature affects. Collision Theory Effect Of Temperature On Reaction Rate.
From www.studypool.com
SOLUTION Effect of temperature on reaction rate collision theory Collision Theory Effect Of Temperature On Reaction Rate It assumes that you are already familiar with basic ideas about the collision theory, and. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the. Collision Theory Effect Of Temperature On Reaction Rate.
From chem.libretexts.org
14.9 The Effect of Temperature on Reaction Rates Chemistry LibreTexts Collision Theory Effect Of Temperature On Reaction Rate Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Diagram showing the increased kinetic energy that particles have at higher temperatures. There are several factors that can affect the rate of a reaction. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature. Collision Theory Effect Of Temperature On Reaction Rate.
From unacademy.com
Effect of Temperature on The Rate of Reaction Collision Theory Effect Of Temperature On Reaction Rate It assumes that you are already familiar with basic ideas about the collision theory, and. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. In this section, we will use the collision model to analyze this relationship between. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. Collision Theory Effect Of Temperature On Reaction Rate.
From www.studypool.com
SOLUTION Effect of temperature on reaction rate collision theory Collision Theory Effect Of Temperature On Reaction Rate For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. There are several factors that can affect the rate of a reaction. The arrhenius equation describes the relation between a reaction’s. It assumes that you are already familiar with basic ideas about the collision theory, and. This page. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Collision theory in the rate of reaction YouTube Collision Theory Effect Of Temperature On Reaction Rate The arrhenius equation describes the relation between a reaction’s. Diagram showing the increased kinetic energy that particles have at higher temperatures. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. In this section, we will use the collision model to analyze this relationship between. It assumes that you are already familiar with basic ideas about. Collision Theory Effect Of Temperature On Reaction Rate.
From www.chemicals.co.uk
Chemistry GCSE Revision The Rate and Extent of Chemical Change Collision Theory Effect Of Temperature On Reaction Rate The collision model explains why chemical reactions often occur more rapidly at higher temperatures. There are several factors that can affect the rate of a reaction. The arrhenius equation describes the relation between a reaction’s. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision theory to. Collision Theory Effect Of Temperature On Reaction Rate.
From www.worthington-biochem.com
Temperature Effects Worthington Biochemical Collision Theory Effect Of Temperature On Reaction Rate The rate of the reaction depends. It assumes that you are already familiar with basic ideas about the collision theory, and. This page describes and explains the way that changing the temperature affects the rate of a reaction. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c.. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Collision Theory and Reaction Rate // HSC Chemistry YouTube Collision Theory Effect Of Temperature On Reaction Rate Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The rate of the reaction depends. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Temperature dependence theory of rate constant Arrhenius Equation Collision Theory Effect Of Temperature On Reaction Rate The arrhenius equation describes the relation between a reaction’s. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of the reaction depends. This page describes and explains the way that changing the temperature affects the rate of a reaction. In this section, we will use the collision model to. Collision Theory Effect Of Temperature On Reaction Rate.
From www.youtube.com
Reaction Rate & Collision Theory // Preliminary HSC Chemistry YouTube Collision Theory Effect Of Temperature On Reaction Rate There are several factors that can affect the rate of a reaction. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The rate of the reaction depends. For example, the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10°c. The collision. Collision Theory Effect Of Temperature On Reaction Rate.
From www.scribd.com
The Effects of Temperature, Concentration, and Surface Area on Reaction Collision Theory Effect Of Temperature On Reaction Rate In this section, we will use the collision model to analyze this relationship between. The collision model explains why chemical reactions often occur more rapidly at higher temperatures. The arrhenius equation describes the relation between a reaction’s. This page describes and explains the way that changing the temperature affects the rate of a reaction. Use the postulates of collision theory. Collision Theory Effect Of Temperature On Reaction Rate.