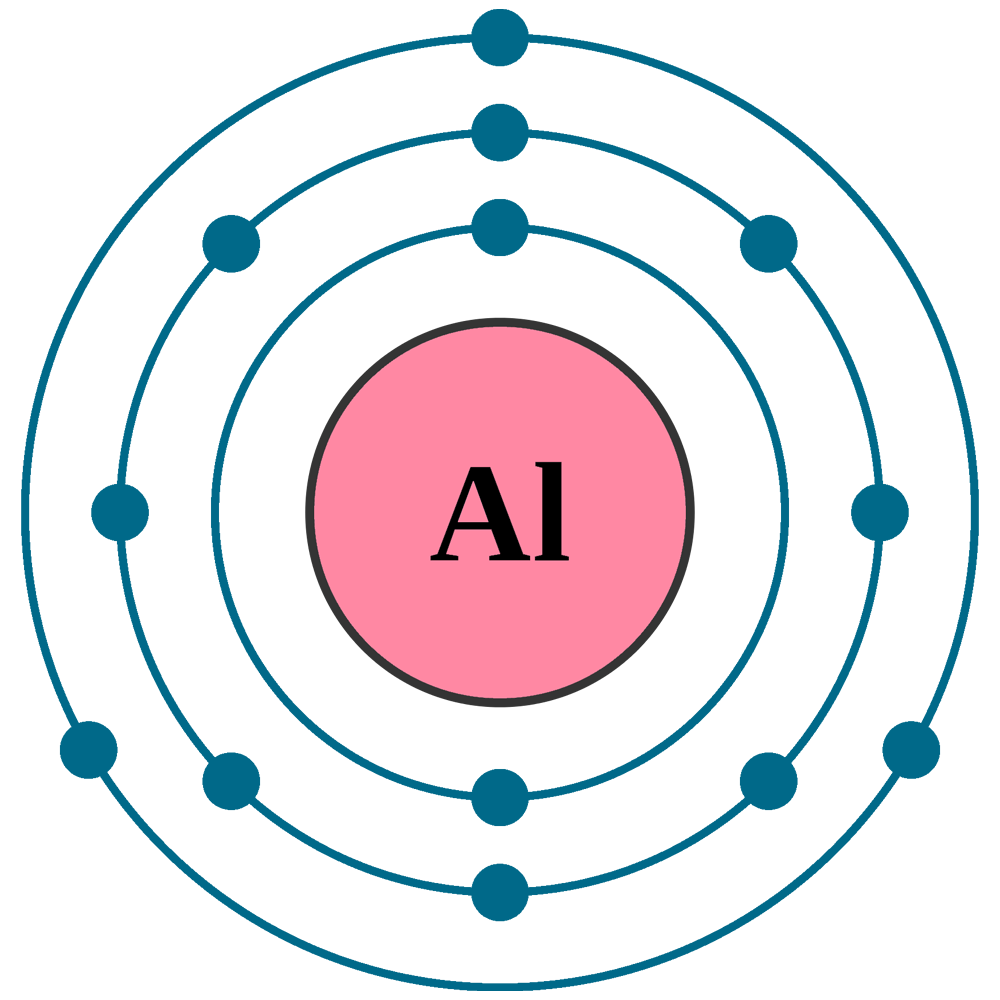
Aluminum Chloride Valence Electrons . The possibility of electrons in its d shell makes it hypervalent. In group 7 of the periodic table, chlorine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Hence, aluminum has three valence electrons and chlorine has seven valence. Total electron pairs are determined by dividing the number total. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. Chlorine’s electronic configuration is given by [ne]3s23p5. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons.
from newtondesk.com
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. In group 7 of the periodic table, chlorine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The possibility of electrons in its d shell makes it hypervalent. Hence, aluminum has three valence electrons and chlorine has seven valence. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. Chlorine’s electronic configuration is given by [ne]3s23p5. Total electron pairs are determined by dividing the number total.
Aluminium Al (Element 13) of Periodic Table Elements FlashCards
Aluminum Chloride Valence Electrons Total electron pairs are determined by dividing the number total. Chlorine’s electronic configuration is given by [ne]3s23p5. Hence, aluminum has three valence electrons and chlorine has seven valence. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. Total electron pairs are determined by dividing the number total. The possibility of electrons in its d shell makes it hypervalent. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. In group 7 of the periodic table, chlorine has seven valence electrons.
From newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards Aluminum Chloride Valence Electrons Total electron pairs are determined by dividing the number total. The possibility of electrons in its d shell makes it hypervalent. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Hence,. Aluminum Chloride Valence Electrons.
From www.anyrgb.com
Aluminium chloride, ionic Compound, Valence electron, Ionic Bonding Aluminum Chloride Valence Electrons Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Total electron pairs are determined by dividing the number total. The possibility of electrons in its d shell makes it hypervalent. Hence, aluminum has three valence electrons and chlorine has seven valence. In group 7 of the periodic table, chlorine has seven valence electrons.. Aluminum Chloride Valence Electrons.
From littleeagles.edu.vn
Aluminum Orbital Diagram, Electron Configuration, And Valence Electrons Aluminum Chloride Valence Electrons In group 7 of the periodic table, chlorine has seven valence electrons. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. The possibility of electrons in its d shell makes it hypervalent. Hence, aluminum has three valence electrons and. Aluminum Chloride Valence Electrons.
From www.youtube.com
How to Draw the Lewis Structure for AlCl3 Aluminum Chloride YouTube Aluminum Chloride Valence Electrons In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. In group 7 of the periodic table, chlorine has seven valence electrons. Chlorine’s electronic configuration is given by [ne]3s23p5. The possibility of electrons in its d shell makes it hypervalent. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. The. Aluminum Chloride Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum (Al) Have? Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The valence electrons of aluminum chloride are the sum of the total valence electrons. Aluminum Chloride Valence Electrons.
From www.youtube.com
Aluminum Electron Configuration YouTube Aluminum Chloride Valence Electrons Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The possibility of electrons in its d shell makes it hypervalent. The valence electrons of aluminum chloride are the sum of the. Aluminum Chloride Valence Electrons.
From www.youtube.com
How many valence electrons does aluminum have?How to find the valence Aluminum Chloride Valence Electrons In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Aluminum Chloride Valence Electrons.
From www.shutterstock.com
Aluminum Chloride Molecule Structure Stock Vector (Royalty Free Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Hence, aluminum has three valence electrons and chlorine has seven valence. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The possibility of electrons in its d shell makes it hypervalent. Therefore, the three chlorine atoms present contributes. Aluminum Chloride Valence Electrons.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Aluminum Chloride Valence Electrons Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. The possibility of electrons in its d shell makes it hypervalent. In group 7 of the periodic table, chlorine has seven valence electrons. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. Chlorine’s electronic configuration is given by. Aluminum Chloride Valence Electrons.
From www.science-revision.co.uk
Dative Covalent bonding Aluminum Chloride Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In group 7 of the periodic table, chlorine has seven valence electrons. Total electron pairs are determined by dividing the number total. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons.. Aluminum Chloride Valence Electrons.
From topblogtenz.com
Aluminum Orbital diagram, Electron configuration, and Valence electrons Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Total electron pairs are determined by dividing the number total. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. The possibility of electrons in its. Aluminum Chloride Valence Electrons.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Aluminum Chloride Valence Electrons Total electron pairs are determined by dividing the number total. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. Total valance electrons pairs = σ bonds + π bonds + lone. Aluminum Chloride Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Aluminum Chloride? Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Hence, aluminum has three valence electrons and chlorine has seven valence. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. The valence electrons of aluminum chloride are the sum of the. Aluminum Chloride Valence Electrons.
From quizizz.com
Review Lesson Ionic Bonds and Naming Science Quizizz Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The possibility of electrons in its d shell makes it hypervalent. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. In group. Aluminum Chloride Valence Electrons.
From www.sciencephoto.com
Aluminum, atomic structure Stock Image C018/3694 Science Photo Aluminum Chloride Valence Electrons Hence, aluminum has three valence electrons and chlorine has seven valence. The possibility of electrons in its d shell makes it hypervalent. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Total electron pairs are determined by dividing the number total. In group 7 of. Aluminum Chloride Valence Electrons.
From circuitmuchlimonnt.z13.web.core.windows.net
Aluminum Electron Dot Diagram Aluminum Chloride Valence Electrons The possibility of electrons in its d shell makes it hypervalent. In group 7 of the periodic table, chlorine has seven valence electrons. Hence, aluminum has three valence electrons and chlorine has seven valence. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. 93 rows you may assume the valences of the chemical. Aluminum Chloride Valence Electrons.
From www.adda247.com
Aluminium Chloride Formula AlCl3 Chemical Compound Name, Structure Aluminum Chloride Valence Electrons In group 7 of the periodic table, chlorine has seven valence electrons. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. The possibility of electrons in its d shell makes it hypervalent. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons.. Aluminum Chloride Valence Electrons.
From ar.inspiredpencil.com
Aluminum Chloride Lewis Structure Aluminum Chloride Valence Electrons The possibility of electrons in its d shell makes it hypervalent. Total electron pairs are determined by dividing the number total. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The valence electrons of aluminum chloride are the sum of the total valence electrons of. Aluminum Chloride Valence Electrons.
From favpng.com
Bohr Model Electron Aluminium Lewis Structure Atom, PNG, 600x590px Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Hence, aluminum has three valence electrons and chlorine has seven valence. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. In group 7 of the periodic table, chlorine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons. Aluminum Chloride Valence Electrons.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Aluminum Chloride Valence Electrons In group 7 of the periodic table, chlorine has seven valence electrons. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. Total electron pairs are determined by dividing the number total. The possibility of electrons in its d shell makes it hypervalent. 93 rows you may assume the valences of the chemical elements—the number. Aluminum Chloride Valence Electrons.
From ar.inspiredpencil.com
Aluminum Chloride Lewis Structure Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The possibility of electrons in its d shell makes it hypervalent. Hence, aluminum has three. Aluminum Chloride Valence Electrons.
From aluminumchloridegoshiema.blogspot.com
Aluminum Chloride Aluminum Chloride Lewis Dot Aluminum Chloride Valence Electrons The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. In group 7 of the periodic table, chlorine has seven valence electrons. In the periodic table, aluminum lies in group 13, and chlorine. Aluminum Chloride Valence Electrons.
From slideplayer.com
Lewis Symbols To help us to focus on the valence electrons those that Aluminum Chloride Valence Electrons Hence, aluminum has three valence electrons and chlorine has seven valence. Chlorine’s electronic configuration is given by [ne]3s23p5. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. Total valance electrons pairs = σ bonds + π bonds + lone pairs. Aluminum Chloride Valence Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Chloride Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Hence, aluminum has three valence electrons and chlorine has seven valence. In group 7 of the periodic table, chlorine has seven valence electrons. The possibility of electrons in its d shell makes it hypervalent. Chlorine’s electronic. Aluminum Chloride Valence Electrons.
From periodictable.me
Aluminum Valence Electrons Aluminum Valency (Al) with Dot Diagram Aluminum Chloride Valence Electrons Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. In group 7 of the periodic table, chlorine has seven valence electrons. Total electron pairs are determined by dividing the number total. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. In. Aluminum Chloride Valence Electrons.
From www.youtube.com
How to Draw the Lewis Structure for AlCl3 Aluminum chloride YouTube Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Total electron pairs are determined by dividing the number total. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. The possibility of electrons in. Aluminum Chloride Valence Electrons.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Aluminum General Wiring Diagram Aluminum Chloride Valence Electrons Total electron pairs are determined by dividing the number total. The possibility of electrons in its d shell makes it hypervalent. Chlorine’s electronic configuration is given by [ne]3s23p5. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Aluminum Chloride Valence Electrons.
From aluminumchloridegoshiema.blogspot.com
Aluminum Chloride Aluminum Chloride Lewis Structure Aluminum Chloride Valence Electrons The possibility of electrons in its d shell makes it hypervalent. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. In group 7 of the periodic table, chlorine has seven valence electrons.. Aluminum Chloride Valence Electrons.
From chemistry291.blogspot.com
How to Draw Al (Aluminum) Lewis Dot Structure Aluminum Chloride Valence Electrons Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Hence, aluminum has three valence electrons and chlorine has seven valence. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The possibility of electrons in its d shell makes. Aluminum Chloride Valence Electrons.
From www.youtube.com
How to Find Valence Electrons for Aluminum (Al) YouTube Aluminum Chloride Valence Electrons Hence, aluminum has three valence electrons and chlorine has seven valence. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. Total electron pairs are determined by dividing the number total.. Aluminum Chloride Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum Chloride Have? Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. In group 7 of the periodic table, chlorine has seven valence electrons. Total valance electrons pairs. Aluminum Chloride Valence Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Aluminium (Al)? Aluminum Chloride Valence Electrons Hence, aluminum has three valence electrons and chlorine has seven valence. The possibility of electrons in its d shell makes it hypervalent. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. Total. Aluminum Chloride Valence Electrons.
From schematicellshastes.z21.web.core.windows.net
Aluminum Electron Dot Diagram Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. Hence, aluminum has three valence electrons and chlorine has seven valence. In the periodic table, aluminum lies in group 13, and chlorine lies in group 17. The possibility of electrons in its d shell makes it hypervalent. 93 rows. Aluminum Chloride Valence Electrons.
From slideplayer.com
Ions, Ionic Bonds, and Metallic Bonds ppt download Aluminum Chloride Valence Electrons Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. 93 rows you may assume the valences. Aluminum Chloride Valence Electrons.
From www.youtube.com
Alcl3 Lewis Structure (Aluminum Chloride) YouTube Aluminum Chloride Valence Electrons Chlorine’s electronic configuration is given by [ne]3s23p5. Therefore, the three chlorine atoms present contributes 7 x 3 = 21 valence electrons. The possibility of electrons in its d shell makes it hypervalent. The valence electrons of aluminum chloride are the sum of the total valence electrons of aluminum and chlorine in the compound alcl 3. Total valance electrons pairs =. Aluminum Chloride Valence Electrons.