Aluminium Atom Size . Aluminum and silicon are both in the third row with aluminum lying to the left, so. Radii of atoms and ions. This periodic table shows the relative sizes of the atoms of each element. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. The atomic radius of aluminium atom is 121pm (covalent radius). Atom size values are calculated. Below mentioned radii are the van der waals radius in picometer (pm)). Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The atomic radius of a chemical element. Each atom’s size is relative to the largest element, cesium. 119 rows atomic radius of all the elements are mentioned in the chart below.
from www.alamy.com
This periodic table shows the relative sizes of the atoms of each element. 119 rows atomic radius of all the elements are mentioned in the chart below. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Atom size values are calculated. The atomic radius of a chemical element. The atomic radius of aluminium atom is 121pm (covalent radius). Each atom’s size is relative to the largest element, cesium. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Below mentioned radii are the van der waals radius in picometer (pm)).
3d render of atom structure of aluminum isolated over white background
Aluminium Atom Size Atom size values are calculated. Radii of atoms and ions. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Aluminum and silicon are both in the third row with aluminum lying to the left, so. This periodic table shows the relative sizes of the atoms of each element. The atomic radius of a chemical element. The atomic radius of aluminium atom is 121pm (covalent radius). Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. 119 rows atomic radius of all the elements are mentioned in the chart below. Each atom’s size is relative to the largest element, cesium. Atom size values are calculated. Below mentioned radii are the van der waals radius in picometer (pm)).
From www.dreamstime.com
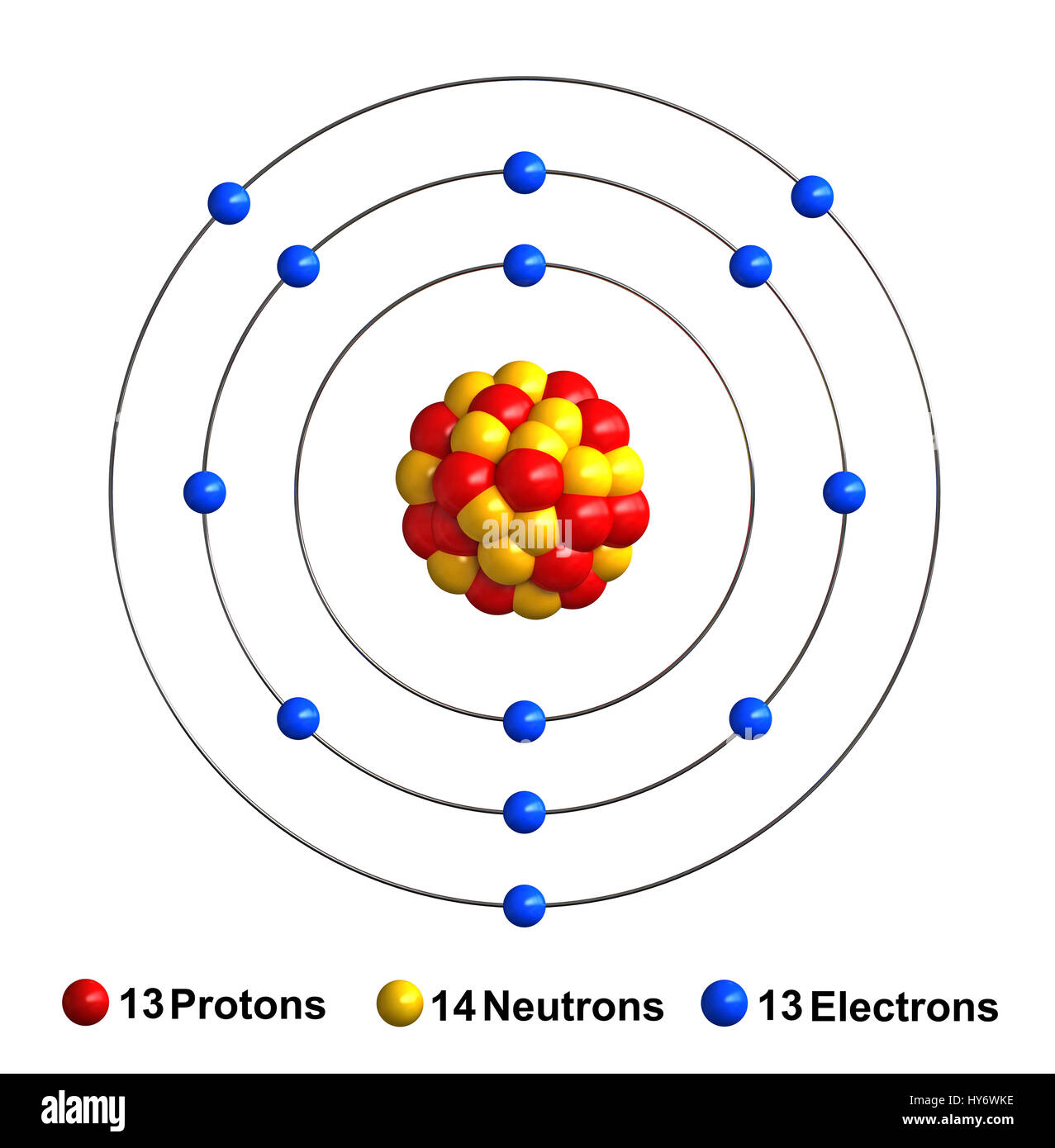
Model of aluminium atom stock vector. Illustration of science 164474877 Aluminium Atom Size This periodic table shows the relative sizes of the atoms of each element. Radii of atoms and ions. Atom size values are calculated. 119 rows atomic radius of all the elements are mentioned in the chart below. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Below mentioned. Aluminium Atom Size.
From www.collegesidekick.com
Atomic Size Introduction to Chemistry Aluminium Atom Size Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Aluminum and silicon are both in the third row with aluminum lying to the left, so. 119 rows atomic radius of all the elements are mentioned in the chart below. The atomic radius of aluminium atom is 121pm (covalent. Aluminium Atom Size.
From material-properties.org
Aluminium Periodic Table and Atomic Properties Aluminium Atom Size Aluminum and silicon are both in the third row with aluminum lying to the left, so. Each atom’s size is relative to the largest element, cesium. The atomic radius of a chemical element. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Below mentioned. Aluminium Atom Size.
From www.sciencephoto.com
Aluminium, atomic structure Stock Image C013/1526 Science Photo Library Aluminium Atom Size Each atom’s size is relative to the largest element, cesium. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Radii of atoms and ions. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Under most definitions the radii of isolated neutral. Aluminium Atom Size.
From www.alamy.com
Aluminium (Al). Diagram of the nuclear composition and electron Aluminium Atom Size The atomic radius of aluminium atom is 121pm (covalent radius). Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. 119 rows atomic radius of all the elements are mentioned in the chart below. Radii of atoms and ions. Aluminum and silicon are both in. Aluminium Atom Size.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Aluminium Atom Size Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The atomic radius of a chemical element. Atom size values are calculated. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Each atom’s. Aluminium Atom Size.
From www.sciencephoto.com
Aluminium, atomic structure Stock Image C019/7644 Science Photo Aluminium Atom Size 119 rows atomic radius of all the elements are mentioned in the chart below. Each atom’s size is relative to the largest element, cesium. Below mentioned radii are the van der waals radius in picometer (pm)). The atomic radius of aluminium atom is 121pm (covalent radius). Radii of atoms and ions. This periodic table shows the relative sizes of the. Aluminium Atom Size.
From www.shutterstock.com
Diagram Aluminum Atom Periodic Table Element Stock Vector (Royalty Free Aluminium Atom Size Each atom’s size is relative to the largest element, cesium. Radii of atoms and ions. The atomic radius of aluminium atom is 121pm (covalent radius). The atomic radius of a chemical element. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Below mentioned radii are the van der waals radius in picometer (pm)).. Aluminium Atom Size.
From www.slideserve.com
PPT Nuclear model of atom PowerPoint Presentation, free download ID Aluminium Atom Size Radii of atoms and ions. Atom size values are calculated. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Below mentioned radii are the van der waals radius in picometer (pm)). 119. Aluminium Atom Size.
From www.slideshare.net
Atomic Theory Aluminium Atom Size 119 rows atomic radius of all the elements are mentioned in the chart below. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si).. Aluminium Atom Size.
From www.alamy.com
Atom model aluminium Stock Vector Images Alamy Aluminium Atom Size The atomic radius of a chemical element. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. The atomic radius of aluminium atom is 121pm (covalent radius). 119 rows. Aluminium Atom Size.
From www.vectorstock.com
Diagram representation of the element aluminium Vector Image Aluminium Atom Size Each atom’s size is relative to the largest element, cesium. Atom size values are calculated. The atomic radius of a chemical element. 119 rows atomic radius of all the elements are mentioned in the chart below. Radii of atoms and ions. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Under most definitions. Aluminium Atom Size.
From www.alamy.com
Aluminum chemical element, Sign with atomic number and atomic weight Aluminium Atom Size Atom size values are calculated. Each atom’s size is relative to the largest element, cesium. 119 rows atomic radius of all the elements are mentioned in the chart below. This periodic table shows the relative sizes of the atoms of each element. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a. Aluminium Atom Size.
From chemwiki.ucdavis.edu
Chapter 3.2 Sizes of Atoms and Ions Chemwiki Aluminium Atom Size The atomic radius of a chemical element. Below mentioned radii are the van der waals radius in picometer (pm)). This periodic table shows the relative sizes of the atoms of each element. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Each atom’s size. Aluminium Atom Size.
From periodictableguide.com
Aluminum (Al) Periodic Table (Element Information & More) Aluminium Atom Size 119 rows atomic radius of all the elements are mentioned in the chart below. Radii of atoms and ions. This periodic table shows the relative sizes of the atoms of each element. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Aluminum and silicon. Aluminium Atom Size.
From www.slideserve.com
PPT Atomic Size PowerPoint Presentation, free download ID6875591 Aluminium Atom Size 119 rows atomic radius of all the elements are mentioned in the chart below. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Aluminum and silicon are both in the third row with aluminum lying to the left, so. This periodic table shows the relative sizes of the. Aluminium Atom Size.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Aluminium Atom Size Each atom’s size is relative to the largest element, cesium. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Radii of atoms and ions. 119 rows atomic radius of all the elements are mentioned in the chart below. Carbon and silicon are both in group 14 with carbon lying above, so carbon is. Aluminium Atom Size.
From www.alamy.com
Icon of the element aluminium of the periodic table with representation Aluminium Atom Size Aluminum and silicon are both in the third row with aluminum lying to the left, so. The atomic radius of aluminium atom is 121pm (covalent radius). Atom size values are calculated. This periodic table shows the relative sizes of the atoms of each element. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller. Aluminium Atom Size.
From www.chem.fsu.edu
Electron Configurations Aluminium Atom Size The atomic radius of aluminium atom is 121pm (covalent radius). Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Below mentioned radii are the van der waals radius in picometer (pm)). Radii of atoms and ions. Aluminum and silicon are both in the third. Aluminium Atom Size.
From www.schoolmykids.com
Aluminium (Al) Element Information, Facts, Properties, Uses Aluminium Atom Size The atomic radius of aluminium atom is 121pm (covalent radius). Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The atomic radius of a chemical element. This periodic table shows the relative sizes of the atoms of each element. 119 rows atomic radius of all the elements are. Aluminium Atom Size.
From www.istockphoto.com
Infographic Of The Element Of Aluminium Stock Illustration Download Aluminium Atom Size Atom size values are calculated. 119 rows atomic radius of all the elements are mentioned in the chart below. Each atom’s size is relative to the largest element, cesium. This periodic table shows the relative sizes of the atoms of each element. The atomic radius of a chemical element. Below mentioned radii are the van der waals radius in picometer. Aluminium Atom Size.
From www.alamy.com
A Aluminium atom diagram Stock Vector Image & Art Alamy Aluminium Atom Size Radii of atoms and ions. Aluminum and silicon are both in the third row with aluminum lying to the left, so. This periodic table shows the relative sizes of the atoms of each element. Each atom’s size is relative to the largest element, cesium. The atomic radius of aluminium atom is 121pm (covalent radius). Carbon and silicon are both in. Aluminium Atom Size.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Aluminium Atom Size Radii of atoms and ions. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Below mentioned radii are the van der waals radius in picometer (pm)). The atomic radius of aluminium atom is 121pm (covalent radius). Under most definitions the radii of isolated neutral atoms range between 30. Aluminium Atom Size.
From webmis.highland.cc.il.us
Sizes of Atoms and Ions Aluminium Atom Size 119 rows atomic radius of all the elements are mentioned in the chart below. Atom size values are calculated. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Below mentioned radii are the van der waals radius in picometer (pm)). Radii of atoms and. Aluminium Atom Size.
From parlonssciences.ca
Introduction au tableau périodique des éléments Parlons sciences Aluminium Atom Size Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Aluminum and silicon are both in the third row with aluminum lying to the left, so. The atomic radius of a chemical element. This periodic table shows the relative sizes of the atoms of each element. Below mentioned radii. Aluminium Atom Size.
From sciencenotes.org
Periodic Table of Element Atom Sizes Aluminium Atom Size Each atom’s size is relative to the largest element, cesium. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Atom size values are calculated. The atomic radius of aluminium atom is 121pm (covalent radius). 119 rows atomic radius of all the elements are mentioned in the chart below. Under most definitions the radii. Aluminium Atom Size.
From saylordotorg.github.io
Sizes of Atoms and Ions Aluminium Atom Size The atomic radius of a chemical element. Below mentioned radii are the van der waals radius in picometer (pm)). Each atom’s size is relative to the largest element, cesium. Radii of atoms and ions. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Atom. Aluminium Atom Size.
From www.slideserve.com
PPT Electron Orbitals PowerPoint Presentation, free download ID2083443 Aluminium Atom Size Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Aluminum and silicon are both in the third row with aluminum lying to the left, so. Each atom’s size is relative to the largest element, cesium. Under most definitions the radii of isolated neutral atoms range between 30 and. Aluminium Atom Size.
From sciencenotes.org
Aluminum Atom Science Notes and Projects Aluminium Atom Size The atomic radius of a chemical element. The atomic radius of aluminium atom is 121pm (covalent radius). 119 rows atomic radius of all the elements are mentioned in the chart below. Below mentioned radii are the van der waals radius in picometer (pm)). Radii of atoms and ions. Each atom’s size is relative to the largest element, cesium. This periodic. Aluminium Atom Size.
From newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards Aluminium Atom Size The atomic radius of a chemical element. Aluminum and silicon are both in the third row with aluminum lying to the left, so. Each atom’s size is relative to the largest element, cesium. Atom size values are calculated. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3. Aluminium Atom Size.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Aluminium Atom Size Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Below mentioned radii are the van der waals radius in picometer (pm)). Radii of atoms and ions. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3. Aluminium Atom Size.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation Aluminium Atom Size Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. This periodic table shows the relative sizes of the atoms of each element. Below mentioned radii are the van der waals radius in picometer (pm)). Carbon and silicon are both in group 14 with carbon. Aluminium Atom Size.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Aluminium Atom Size Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. The atomic radius of a chemical element. This periodic table shows the relative sizes of the atoms of each element. Carbon and silicon are both in group 14 with carbon lying above, so carbon is. Aluminium Atom Size.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Aluminium Atom Size Aluminum and silicon are both in the third row with aluminum lying to the left, so. The atomic radius of aluminium atom is 121pm (covalent radius). Atom size values are calculated. Radii of atoms and ions. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). This periodic table. Aluminium Atom Size.
From sciencenotes.org
Atomic Radius and Ionic Radius Aluminium Atom Size Below mentioned radii are the van der waals radius in picometer (pm)). Atom size values are calculated. This periodic table shows the relative sizes of the atoms of each element. Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. 119 rows atomic radius of. Aluminium Atom Size.