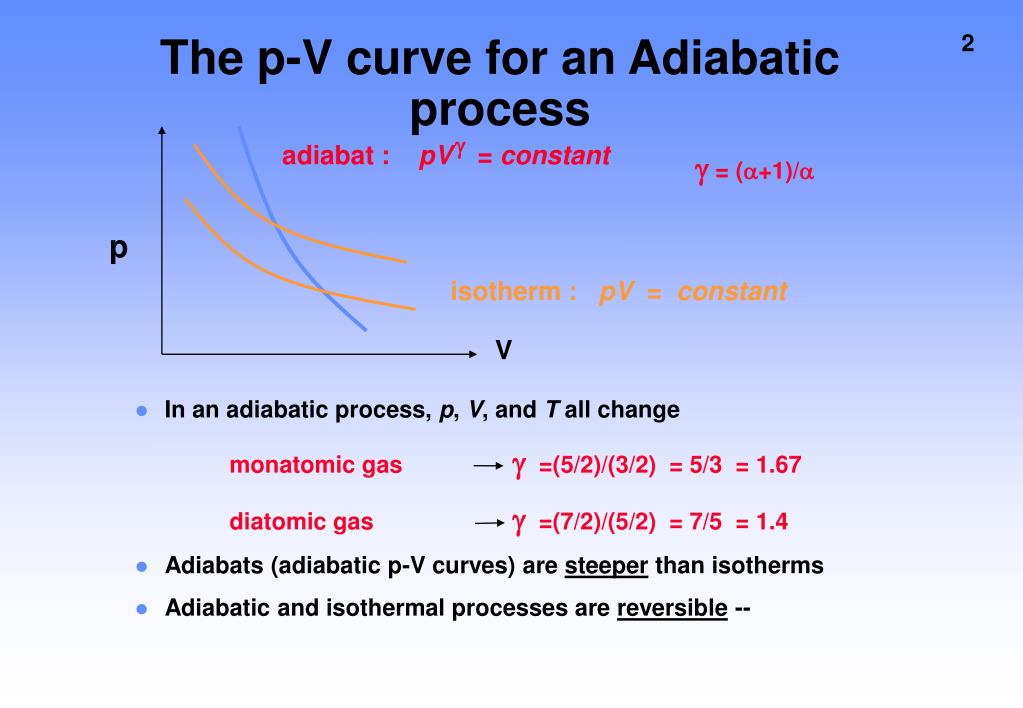
Calculate Reversible Adiabatic . For an ideal gas (one mole) t. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. And in an adiabatic compression (v2 < v1), the gas heats up. In a reversible adiabatic process: Demonstrate the qualitative difference between adiabatic and isothermal expansions. Define adiabatic expansion of an ideal gas. Thus we can imagine a gas in a closed. Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. Calculate the final pressure when a sample of he at 250. In this combined gas law calculator, we consider processes in which the number of particles is constant.
from www.slideserve.com
From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. In a reversible adiabatic process: Calculate the final pressure when a sample of he at 250. In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). For an ideal gas (one mole) t. Thus we can imagine a gas in a closed. Demonstrate the qualitative difference between adiabatic and isothermal expansions. And in an adiabatic compression (v2 < v1), the gas heats up. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant.
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint
Calculate Reversible Adiabatic And in an adiabatic compression (v2 < v1), the gas heats up. And in an adiabatic compression (v2 < v1), the gas heats up. Calculate the final pressure when a sample of he at 250. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. In a reversible adiabatic process: Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. In this combined gas law calculator, we consider processes in which the number of particles is constant. From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. Demonstrate the qualitative difference between adiabatic and isothermal expansions. For an ideal gas (one mole) t. Define adiabatic expansion of an ideal gas. Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Thus we can imagine a gas in a closed.
From byjus.com
Work done in adiabatic process Calculate Reversible Adiabatic From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. In this combined gas law calculator, we consider processes in which the number of particles is constant. Define adiabatic expansion of an ideal gas. Demonstrate the qualitative difference between adiabatic and isothermal expansions. For an ideal gas (one mole) t. Thus we can. Calculate Reversible Adiabatic.
From www.toppr.com
Work done in reversible adiabatic process by an ideal gas is given by Calculate Reversible Adiabatic Calculate the final pressure when a sample of he at 250. From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. For an ideal gas (one mole) t. Let us calculate the work done by a mole of an ideal. Calculate Reversible Adiabatic.
From learncheme.com
LearnChemE Calculate Reversible Adiabatic In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. In a reversible adiabatic process: Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. And in an adiabatic compression (v2 <. Calculate Reversible Adiabatic.
From www.numerade.com
SOLVED 0.2 m^3 of air at 4 bar and 13°C is contained in system A Calculate Reversible Adiabatic Calculate the final pressure when a sample of he at 250. Demonstrate the qualitative difference between adiabatic and isothermal expansions. Thus we can imagine a gas in a closed. For an ideal gas (one mole) t. In a reversible adiabatic process: In this combined gas law calculator, we consider processes in which the number of particles is constant. Define adiabatic. Calculate Reversible Adiabatic.
From www.chemistrylearner.com
Adiabatic Process Definition, Examples, and Equations Calculate Reversible Adiabatic Define adiabatic expansion of an ideal gas. In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Demonstrate the qualitative difference between adiabatic and isothermal expansions. Calculate the final pressure when a sample of he at 250. Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. In a reversible adiabatic process: For an ideal. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Calculate Reversible Adiabatic In a reversible adiabatic process: From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. And in an adiabatic compression (v2 < v1), the gas heats up. Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2):. Calculate Reversible Adiabatic.
From www.youtube.com
Thermodynamics 8 (Work done in adiabatic process) YouTube Calculate Reversible Adiabatic In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). And in an adiabatic compression (v2 < v1), the gas heats up. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. In this combined gas law calculator, we consider processes in which the number. Calculate Reversible Adiabatic.
From www.chegg.com
Solved Show That (T1/T2)=(P2/P1) For A Reversible Adiabat... Calculate Reversible Adiabatic In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. Demonstrate the qualitative difference between adiabatic and isothermal expansions. Calculate the final pressure when a sample of he at 250. For an ideal gas (one mole) t. And in an adiabatic compression (v2 <. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Calculate Reversible Adiabatic In a reversible adiabatic process: In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). For an ideal gas (one mole) t. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. Thus we can imagine a gas in a closed. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible. Calculate Reversible Adiabatic.
From www.studocu.com
Isentropic or Reversible Adiabatic Process Adiabatic simply means no Calculate Reversible Adiabatic In this combined gas law calculator, we consider processes in which the number of particles is constant. Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Thus we can imagine a gas in a closed. And in an adiabatic compression (v2 < v1), the gas heats up.. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Calculate Reversible Adiabatic Demonstrate the qualitative difference between adiabatic and isothermal expansions. Calculate the final pressure when a sample of he at 250. From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Kpa. Calculate Reversible Adiabatic.
From www.youtube.com
Thermodynamics reversible adiabatic expansion, colorcoded derivation Calculate Reversible Adiabatic Demonstrate the qualitative difference between adiabatic and isothermal expansions. Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. In this combined gas law calculator, we consider processes in which the number of particles is constant. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant.. Calculate Reversible Adiabatic.
From www.youtube.com
Work done in adiabatic process YouTube Calculate Reversible Adiabatic Thus we can imagine a gas in a closed. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. Let us calculate the work done by a mole of an ideal gas in a reversible. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Calculate Reversible Adiabatic Thus we can imagine a gas in a closed. Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial. Calculate Reversible Adiabatic.
From www.numerade.com
Consider the cycle depicted in the figure below the process AB is a Calculate Reversible Adiabatic In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). In this combined gas law calculator, we consider processes in which the number of particles is constant. Demonstrate the qualitative difference between adiabatic and isothermal expansions. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. Kpa undergoes a reversible adiabatic expansion from 2.00. Calculate Reversible Adiabatic.
From slideplayer.com
Chapter 2 The First Law Unit 3 adiabatic process ppt download Calculate Reversible Adiabatic And in an adiabatic compression (v2 < v1), the gas heats up. In this combined gas law calculator, we consider processes in which the number of particles is constant. Thus we can imagine a gas in a closed. Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. In a reversible adiabatic process: For an ideal gas (one. Calculate Reversible Adiabatic.
From askfilo.com
The reversible expansion of an ideal gas under adiabatic reversible and i.. Calculate Reversible Adiabatic From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Calculate the final pressure when a sample of he at 250. In this combined gas law calculator, we consider processes in which the number of particles is constant. Let us calculate the work done by a mole of an ideal gas in a. Calculate Reversible Adiabatic.
From www.toppr.com
The magnitude of work done by an ideal gas in reversible adiabatic Calculate Reversible Adiabatic In this combined gas law calculator, we consider processes in which the number of particles is constant. In a reversible adiabatic process: Thus we can imagine a gas in a closed. For an ideal gas (one mole) t. Demonstrate the qualitative difference between adiabatic and isothermal expansions. Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial. Calculate Reversible Adiabatic.
From www.numerade.com
SOLVED A monatomic ideal gas expands in a reversible adiabatic process Calculate Reversible Adiabatic Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Calculate the final pressure when a sample of he at 250. For. Calculate Reversible Adiabatic.
From 88guru.com
Adiabatic Process Definition, Equation, Reversible 88Guru Calculate Reversible Adiabatic Define adiabatic expansion of an ideal gas. Calculate the final pressure when a sample of he at 250. In this combined gas law calculator, we consider processes in which the number of particles is constant. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. From these it is easy to see that the ratios of the. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT The Adiabatic Expansion of an Ideal Gas PowerPoint Presentation Calculate Reversible Adiabatic From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Define adiabatic expansion of an ideal gas. In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. Kpa. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Calculate Reversible Adiabatic In this combined gas law calculator, we consider processes in which the number of particles is constant. In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. Demonstrate the qualitative difference between adiabatic and isothermal expansions. For an ideal gas (one mole) t. In. Calculate Reversible Adiabatic.
From scoop.eduncle.com
An adiabatic process is an Calculate Reversible Adiabatic Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. In this combined gas law calculator, we consider processes in which the number of particles is constant. In a reversible adiabatic process: Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Define adiabatic expansion of. Calculate Reversible Adiabatic.
From www.youtube.com
Calculate the final temperature of a monoatomic idal gas that is Calculate Reversible Adiabatic Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. In a reversible adiabatic process: In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial. Calculate Reversible Adiabatic.
From www.youtube.com
Relation between Pressure, Volume and Temperature in Adiabatic Process Calculate Reversible Adiabatic Demonstrate the qualitative difference between adiabatic and isothermal expansions. Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1).. Calculate Reversible Adiabatic.
From learncheme.com
LearnChemE Calculate Reversible Adiabatic Thus we can imagine a gas in a closed. For an ideal gas (one mole) t. Calculate the final pressure when a sample of he at 250. From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Calculate Reversible Adiabatic In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. For an ideal gas (one mole) t. Define. Calculate Reversible Adiabatic.
From www.chegg.com
Solved Calculate ΔU for the reversible adiabatic Calculate Reversible Adiabatic And in an adiabatic compression (v2 < v1), the gas heats up. Define adiabatic expansion of an ideal gas. Calculate the final pressure when a sample of he at 250. Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. Let us calculate the work done by a. Calculate Reversible Adiabatic.
From www.youtube.com
Adiabatic Process 1 YouTube Calculate Reversible Adiabatic Thus we can imagine a gas in a closed. From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\),. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Calculate Reversible Adiabatic Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. In a reversible adiabatic process: Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a. Calculate Reversible Adiabatic.
From adpokat.github.io
What Is Adiabatic Process Calculate Reversible Adiabatic In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). In a reversible adiabatic process: Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. In this combined. Calculate Reversible Adiabatic.
From www.youtube.com
Adiabatic Reversible Process For Ideal Gas YouTube Calculate Reversible Adiabatic For an ideal gas (one mole) t. And in an adiabatic compression (v2 < v1), the gas heats up. From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. In an adiabatic expansion (v2 > v1), the gas cools (t2 > t1). Let us calculate the work done by a mole of an. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Calculate Reversible Adiabatic Kpa undergoes a reversible adiabatic expansion from 2.00 l to 4.00 l. Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): From these it is easy to see that the ratios of the adiabatic, isothermal, isobaric and isochoric. Calculate the final pressure when. Calculate Reversible Adiabatic.
From www.numerade.com
SOLVED Calculate the final temperature, the work done, and the change Calculate Reversible Adiabatic Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): Thus we can imagine a gas in a closed. Pvγ = constant,. Calculate Reversible Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Calculate Reversible Adiabatic Lecture notes on reversible adiabatic expansion (or compression) of an ideal gas, irreversible adiabatic expansion of an ideal gas against a constant. Consider an ideal gas that undergoes a reversible adiabatic expansion from an initial state, specified by known values \(v_1\) and \(t_1\), to. Pvγ = constant, tvγ − 1 = constant, p1 − γtγ = constant. In this combined. Calculate Reversible Adiabatic.