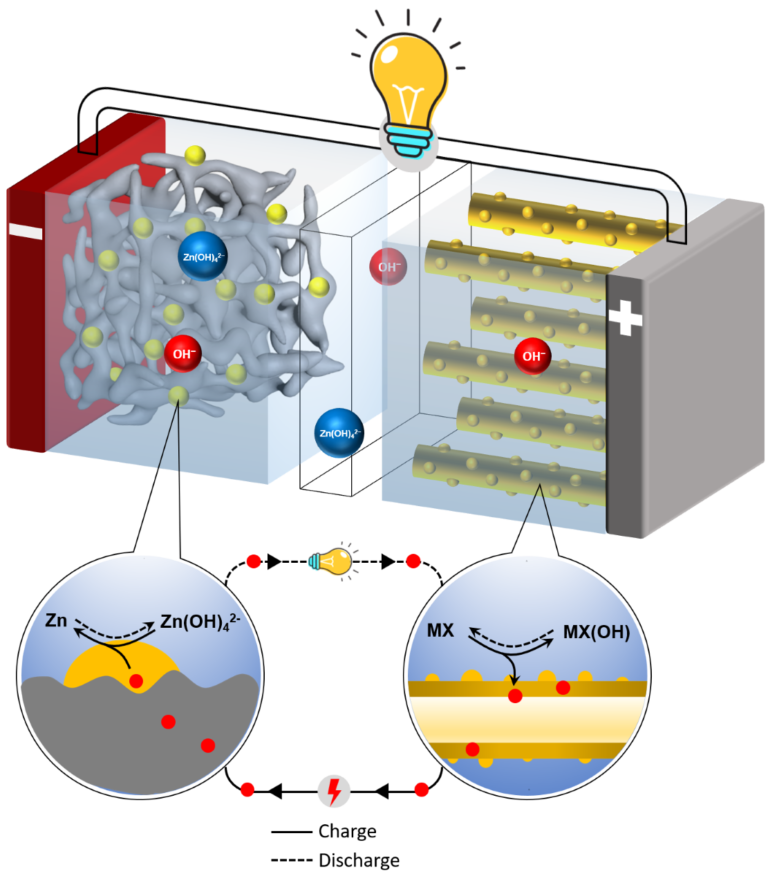
Copper And Zinc Battery . A coordinating hydrogel is used as electrolyte. • a coordinating hydrogel is used as electrolyte. the solution that the researchers present in their current study is therefore based on a simple concept: Recharging the batteries means reversing the flow of current, causing. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. the reaction releases electrons that flow from anode to cathode through an external circuit. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. The use of an alkaline solution. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc.
from www.pv-magazine.com
A coordinating hydrogel is used as electrolyte. the reaction releases electrons that flow from anode to cathode through an external circuit. Recharging the batteries means reversing the flow of current, causing. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. the solution that the researchers present in their current study is therefore based on a simple concept: the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. The use of an alkaline solution. • a coordinating hydrogel is used as electrolyte.
Superfast, longlife aqueous rechargeable zinc battery pv magazine
Copper And Zinc Battery • a coordinating hydrogel is used as electrolyte. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A coordinating hydrogel is used as electrolyte. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. The use of an alkaline solution. the reaction releases electrons that flow from anode to cathode through an external circuit. • a coordinating hydrogel is used as electrolyte. the solution that the researchers present in their current study is therefore based on a simple concept: Recharging the batteries means reversing the flow of current, causing.
From www.slideserve.com
PPT Copper and zinc battery PowerPoint Presentation, free download Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. The use of an alkaline solution. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two. Copper And Zinc Battery.
From www.electricity-magnetism.org
Zincchloride battery extraheavyduty Electricity Copper And Zinc Battery Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. the solution that the researchers present in their current study is therefore based on a simple concept: the daniell cell (zinc copper battery) was only used as primary cell due to the. Copper And Zinc Battery.
From www.aliexpress.com
60pcs/lot Pkcell 1.5V Zinc Carbon Battery AAA R03P UM4 Heavy Duty Dry Copper And Zinc Battery • a coordinating hydrogel is used as electrolyte. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. the solution that the researchers present in their current study is therefore based on a simple concept: the reaction releases electrons that flow from anode to cathode through an external. Copper And Zinc Battery.
From ar.inspiredpencil.com
Zinc Battery Copper And Zinc Battery the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A coordinating hydrogel is used as electrolyte. The use of an alkaline solution. Recharging the batteries means reversing the flow of current, causing. the daniell cell (zinc copper battery) was only used as primary cell due to the copper. Copper And Zinc Battery.
From www.doitpoms.ac.uk
Zinc/carbon batteries Copper And Zinc Battery the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. the reaction releases electrons that flow from anode to cathode through an external circuit. • a coordinating hydrogel is used as electrolyte. A coordinating hydrogel is used as electrolyte. Recharging the batteries means reversing the flow of current, causing.. Copper And Zinc Battery.
From www.pv-magazine.com
Superfast, longlife aqueous rechargeable zinc battery pv magazine Copper And Zinc Battery The use of an alkaline solution. A coordinating hydrogel is used as electrolyte. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its.. Copper And Zinc Battery.
From ar.inspiredpencil.com
Zinc Battery Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: • a coordinating hydrogel is used as electrolyte. The use of an alkaline solution. Recharging the batteries means reversing the flow of current, causing. the reaction releases electrons that flow from anode to cathode through an external circuit. Zinc dissolves in. Copper And Zinc Battery.
From www.britannica.com
Battery Primary Cells, Rechargeable, Chemistry Britannica Copper And Zinc Battery Recharging the batteries means reversing the flow of current, causing. The use of an alkaline solution. A coordinating hydrogel is used as electrolyte. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two. Copper And Zinc Battery.
From en.ppt-online.org
Primary Batteries. The zinccarbon cell online presentation Copper And Zinc Battery the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. • a coordinating hydrogel is used as electrolyte. Recharging the batteries means reversing the flow of current, causing. The use of an alkaline solution. the solution that the researchers present in their current study is therefore based on. Copper And Zinc Battery.
From my.cytron.io
Copper & Zinc Plate Fruit Battery Experiment Kit Copper And Zinc Battery A coordinating hydrogel is used as electrolyte. Recharging the batteries means reversing the flow of current, causing. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. The use. Copper And Zinc Battery.
From nhenhenhem.com
A Battery That's Tough Enough To Take Structural Loads Nhenhenhem Copper And Zinc Battery the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. the solution that the researchers present in their current study is therefore based on a simple concept: A coordinating hydrogel is used as electrolyte. the reaction releases electrons that flow from anode to cathode through an external. Copper And Zinc Battery.
From scitoys.com
Homemade Batteries Science Toys Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A coordinating hydrogel is used as electrolyte. • a coordinating hydrogel is used as electrolyte. The. Copper And Zinc Battery.
From pixels.com
Zinccopper Battery Photograph by Andrew Lambert Photography Copper And Zinc Battery Recharging the batteries means reversing the flow of current, causing. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. • a coordinating hydrogel is used as electrolyte. A coordinating hydrogel is used as electrolyte. the solution that the researchers present in their current study is therefore based. Copper And Zinc Battery.
From news.ultrabookbatteries.com
Zincion Batteries A Sustainable Solution to Climate Change Copper And Zinc Battery Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. the solution that the researchers present in their current study is therefore based on a simple concept: The use of an alkaline solution. • a coordinating hydrogel is used as electrolyte. A coordinating. Copper And Zinc Battery.
From www.nytimes.com
How Zinc Batteries Could Change Energy Storage The New York Times Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: The use of an alkaline solution. A coordinating hydrogel is used as electrolyte. • a coordinating hydrogel is used as electrolyte. Recharging the batteries means reversing the flow of current, causing. the circuit is a loop through the zinc, the wire,. Copper And Zinc Battery.
From www.youtube.com
Salt Water battery kit with Copper and Zinc plates YouTube Copper And Zinc Battery A coordinating hydrogel is used as electrolyte. The use of an alkaline solution. the solution that the researchers present in their current study is therefore based on a simple concept: Recharging the batteries means reversing the flow of current, causing. the reaction releases electrons that flow from anode to cathode through an external circuit. the circuit is. Copper And Zinc Battery.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Copper And Zinc Battery the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. the solution that the researchers present in their current study is therefore based on a simple concept: Recharging the batteries means reversing the flow of current, causing. the reaction releases electrons that flow from anode to cathode through. Copper And Zinc Battery.
From schematicraschizu.z21.web.core.windows.net
Voltaic Cell Diagram Copper And Zinc Battery A coordinating hydrogel is used as electrolyte. Recharging the batteries means reversing the flow of current, causing. • a coordinating hydrogel is used as electrolyte. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. the solution that the researchers present in their current study is therefore based on. Copper And Zinc Battery.
From www.underwoodsolution.com
Carbon zinc D battery,2pcs/Blister Underwood Solution Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: The use of an alkaline solution. • a coordinating hydrogel is used as electrolyte. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. Recharging the batteries means reversing the flow of. Copper And Zinc Battery.
From mydiagram.online
[DIAGRAM] Aufbau Diagram Copper Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A coordinating hydrogel is used as electrolyte. The use of an alkaline solution. Recharging the batteries means reversing the flow of current,. Copper And Zinc Battery.
From www.advancedsciencenews.com
A New Solution to an Old Challenge Recharging CuZn Batteries Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: The use of an alkaline solution. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. • a coordinating hydrogel is used as electrolyte. the reaction releases electrons that flow. Copper And Zinc Battery.
From cosmosmagazine.com
How zinc batteries could power the sustainable economy Copper And Zinc Battery The use of an alkaline solution. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. • a coordinating hydrogel is used as electrolyte. A coordinating hydrogel is used as electrolyte. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to. Copper And Zinc Battery.
From ar.inspiredpencil.com
Zinc Battery Copper And Zinc Battery the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. Recharging the batteries means reversing the flow of current, causing. the reaction releases electrons that flow from anode. Copper And Zinc Battery.
From www.electricity-magnetism.org
Characteristics of Zincair Batteries Voltage, Capacity & Selfdischarge Copper And Zinc Battery A coordinating hydrogel is used as electrolyte. Recharging the batteries means reversing the flow of current, causing. The use of an alkaline solution. the solution that the researchers present in their current study is therefore based on a simple concept: • a coordinating hydrogel is used as electrolyte. the circuit is a loop through the zinc, the wire,. Copper And Zinc Battery.
From scitoys.com
Chapter 3 Electrochemistry Make homemade batteries in your kitchen Copper And Zinc Battery The use of an alkaline solution. the solution that the researchers present in their current study is therefore based on a simple concept: the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two. Copper And Zinc Battery.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Copper And Zinc Battery Recharging the batteries means reversing the flow of current, causing. The use of an alkaline solution. the solution that the researchers present in their current study is therefore based on a simple concept: • a coordinating hydrogel is used as electrolyte. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to. Copper And Zinc Battery.
From www.slideserve.com
PPT Copper and zinc battery PowerPoint Presentation, free download Copper And Zinc Battery The use of an alkaline solution. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. the reaction releases electrons that flow from anode to cathode through an external circuit. the daniell cell (zinc copper battery) was only used as primary cell. Copper And Zinc Battery.
From www.sliderbase.com
Electrochemical Terminology Copper And Zinc Battery A coordinating hydrogel is used as electrolyte. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. the solution that the researchers present in their current study is therefore based on a simple concept: • a coordinating hydrogel is used as electrolyte. Recharging the batteries means reversing the. Copper And Zinc Battery.
From employees.csbsju.edu
Reactivity redox batteries Copper And Zinc Battery A coordinating hydrogel is used as electrolyte. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. Recharging the batteries means reversing the flow of current, causing. the reaction releases electrons that flow from anode to cathode through an external circuit. the. Copper And Zinc Battery.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Copper And Zinc Battery the reaction releases electrons that flow from anode to cathode through an external circuit. • a coordinating hydrogel is used as electrolyte. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A coordinating hydrogel is used as electrolyte. the solution that. Copper And Zinc Battery.
From www.mdpi.com
Batteries Free FullText Secondary ZincAir Batteries A View on Copper And Zinc Battery the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. the reaction releases electrons that flow from anode to cathode through an external circuit. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward. Copper And Zinc Battery.
From www.researchgate.net
(PDF) CopperZinc Battery’s Performance with Different Electrolytes Copper And Zinc Battery Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. • a coordinating hydrogel is used as electrolyte. the circuit is a. Copper And Zinc Battery.
From www.youtube.com
Wire Coil Zinc Copper Acid Battery YouTube Copper And Zinc Battery Recharging the batteries means reversing the flow of current, causing. the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. the solution that the researchers present in their current study is therefore based on a simple concept: the reaction releases electrons that flow from anode to cathode. Copper And Zinc Battery.
From www.takomabattery.com
Zinc manganese battery types and application The Best lithium ion Copper And Zinc Battery the solution that the researchers present in their current study is therefore based on a simple concept: the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. the circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. . Copper And Zinc Battery.
From scitoys.com
Chapter 3 Electrochemistry Make homemade batteries in your kitchen Copper And Zinc Battery • a coordinating hydrogel is used as electrolyte. the solution that the researchers present in their current study is therefore based on a simple concept: the daniell cell (zinc copper battery) was only used as primary cell due to the copper ion crossover preventing its. the reaction releases electrons that flow from anode to cathode through an. Copper And Zinc Battery.