The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That . The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. The potential of standard hydrogen electrode is zero. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. B) electrode potential is considered as zero. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. The standard hydrogen electrode potential is zero because: It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. Δgf ∘ (h +,aq) = 0. 1968 143 amu amu 2009 report error. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We call this e0 e 0, the standard reduction potential.
from www.semanticscholar.org
Δgf ∘ (h +,aq) = 0. 1968 143 amu amu 2009 report error. B) electrode potential is considered as zero. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. We call this e0 e 0, the standard reduction potential. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. The potential of standard hydrogen electrode is zero.
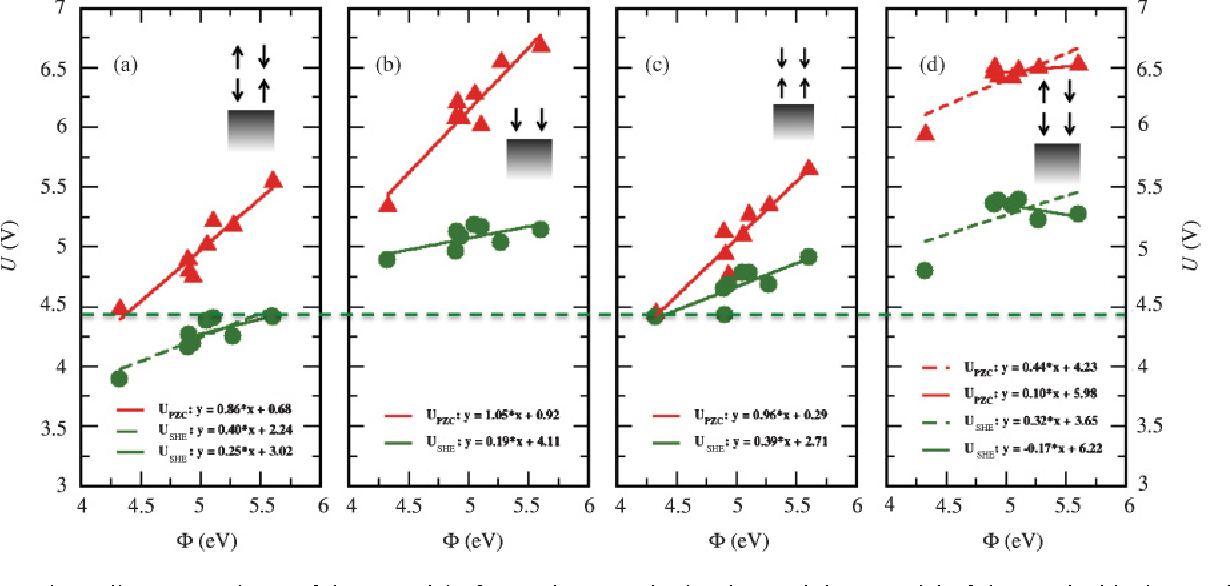
Figure 6 from Standard hydrogen electrode and potential of zero charge
The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That We call this e0 e 0, the standard reduction potential. Δgf ∘ (h +,aq) = 0. B) electrode potential is considered as zero. We call this e0 e 0, the standard reduction potential. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. The standard hydrogen electrode potential is zero because: More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. The potential of standard hydrogen electrode is zero. 1968 143 amu amu 2009 report error. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero.
From www.savemyexams.com
Standard Electrode Potential Edexcel International A Level Chemistry The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That B) electrode potential is considered as zero. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. The standard hydrogen electrode potential is zero because: 1968 143. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That We call this e0 e 0, the standard reduction potential. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. Δgf ∘ (h +,aq) = 0. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. Under. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The standard hydrogen electrode potential is zero because: More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. If standard. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From solvedlib.com
3 Draw a labeled diagram of standard hydrogen electro… SolvedLib The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. 1968 143 amu amu 2009 report error. B) electrode potential is considered as zero. It has been concluded that the standard potential of hydrogen electrode is. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.semanticscholar.org
Figure 3 from Standard hydrogen electrode and potential of zero charge The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The potential of standard hydrogen electrode is zero. Δgf ∘ (h +,aq) = 0. 1968 143 amu amu 2009 report error. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. B) electrode. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The standard hydrogen electrode potential is zero because: The potential of standard hydrogen electrode is zero. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. B) electrode potential is considered as zero. 1968 143 amu amu 2009 report error. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The standard hydrogen electrode potential is zero because: The potential of standard hydrogen electrode is zero. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. B). The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.toppr.com
Which of the following will act as a cathode when connected to a The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. B) electrode potential is considered as zero. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. If standard free energy change of a. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The potential of standard hydrogen electrode is zero. 1968 143 amu amu 2009 report error. We call this e0 e 0, the standard reduction potential. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The potential of standard hydrogen electrode is zero. B) electrode potential is considered as zero. The standard hydrogen electrode potential is zero because: If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. B) electrode potential is considered as zero. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. More correctly, the thermodynamic activity of h + in dilute. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From pandai.me
Standard Electrode Potential The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. Δgf ∘ (h +,aq) = 0. The potential of standard hydrogen electrode is zero. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. The standard hydrogen. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. 1968 143 amu amu 2009 report error. Δgf ∘ (h +,aq) = 0. The standard hydrogen electrode potential is zero because: We call this e0 e 0, the standard reduction potential. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero,. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From question.pandai.org
Standard Electrode Potential The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That 1968 143 amu amu 2009 report error. The potential of standard hydrogen electrode is zero. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. Δgf ∘ (h +,aq) = 0. We call this e0 e 0, the standard reduction potential. It has been concluded that the. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The potential of standard hydrogen electrode is zero. B) electrode potential is considered as zero. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. The standard hydrogen electrode potential is zero because: Δgf ∘ (h +,aq) = 0. Under these conditions, the. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That 1968 143 amu amu 2009 report error. The potential of standard hydrogen electrode is zero. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. To solve. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.toppr.com
A standard hydrogen electrode has zero electrode potential because The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode potential is zero because: Δgf ∘ (h +,aq) = 0. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. We call this e0 e 0, the standard reduction. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From byjus.com
What is standard hydrogen electrode? The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That B) electrode potential is considered as zero. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. We call this e0 e 0, the standard reduction potential. The potential of standard hydrogen electrode is zero. 1968 143 amu amu 2009 report error. Under these conditions, the potential. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.toppr.com
The standard hydrogen electrode has zero electrode potential because The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. We call this e0 e 0, the standard reduction potential. B) electrode potential is. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From studylib.net
Standard Electrode Potentials The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That B) electrode potential is considered as zero. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. We call this. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From monomole.com
Standard hydrogen electrode Mono Mole The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That We call this e0 e 0, the standard reduction potential. The potential of standard hydrogen electrode is zero. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. Δgf ∘ (h +,aq) = 0. To solve the question regarding the standard hydrogen electrode. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That We call this e0 e 0, the standard reduction potential. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. 1968 143 amu amu 2009 report error. Δgf ∘ (h +,aq) = 0. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions,. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From cemubadx.blob.core.windows.net
Standard Cell Potential And Standard Electrode Potential at Nina The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. The standard hydrogen electrode potential is zero because: If standard free energy change of. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.studocu.com
Lecture 5 The measurement of standard potentials The potential of The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. The potential of standard hydrogen electrode is zero. The standard hydrogen electrode potential is zero because: To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From slideplayer.com
Electrochemistry. ppt download The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That B) electrode potential is considered as zero. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. If standard free. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.semanticscholar.org
Figure 6 from Standard hydrogen electrode and potential of zero charge The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That We call this e0 e 0, the standard reduction potential. Δgf ∘ (h +,aq) = 0. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.pinterest.com
Standard Hydrogen Electrode in 2020 Electron configuration, Ideal The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. If standard free energy change of a reaction is zero, this implies that equilibrium. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.youtube.com
Standard Reduction Potentials and the Standard Hydrogen Electrode The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. To solve the question regarding the standard hydrogen electrode (she) and its potential being zero, we can break it down into the following. Δgf ∘ (h +,aq) = 0. The standard hydrogen electrode potential is zero because: It has been. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That We call this e0 e 0, the standard reduction potential. Δgf ∘ (h +,aq) = 0. 1968 143 amu amu 2009 report error. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. To solve the question regarding the standard hydrogen electrode (she) and its potential being. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.youtube.com
Class 12 Electrochemistry / How electrode potential is measured by The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That Δgf ∘ (h +,aq) = 0. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. B) electrode potential is considered as zero. The standard hydrogen electrode refers to any electrode involving h+ ions at standard. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From loeozfjbx.blob.core.windows.net
Nernst Equation For Standard Hydrogen Electrode at Miguel White blog The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That We call this e0 e 0, the standard reduction potential. It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. The standard hydrogen electrode refers to any electrode involving h+ ions at standard conditions, each set to a potential value of 0 v. If standard free energy. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That The standard hydrogen electrode potential is zero because: It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. If standard. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From mungfali.com
Electrode Potential Series The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That It has been concluded that the standard potential of hydrogen electrode is actually zero, as the measured emf value for the cell zn/zncl2. If standard free energy change of a reaction is zero, this implies that equilibrium constant of the reaction is unity. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We call this. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That Δgf ∘ (h +,aq) = 0. More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The potential of standard hydrogen electrode is zero. The standard hydrogen electrode refers. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.
From www.youtube.com
Calculate the potential of hydrogen electrode in contact with a The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That Δgf ∘ (h +,aq) = 0. The standard hydrogen electrode potential is zero because: More correctly, the thermodynamic activity of h + in dilute solution should be replaced by [h +]/c 0, where the standard state concentration c 0 = 1. 1968 143 amu amu 2009 report error. We call this e0 e 0, the standard reduction potential. It has. The Potential Of Standard Hydrogen Electrode Is Zero. This Implies That.