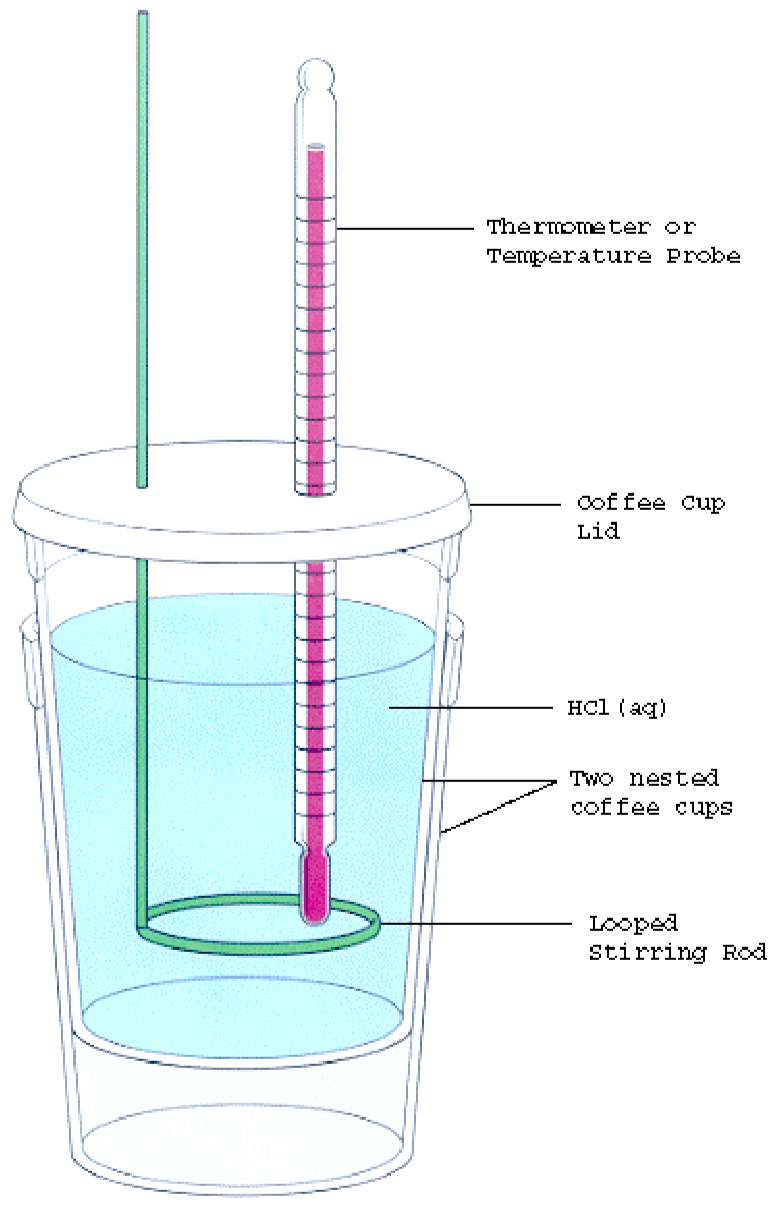
Calorimetry Lab Ideas . See notes 5, 6 and 7 below for additional guidance. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Read our standard health and safety guidance. In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! Complete calculations to find the total mass of hot plus cold water. Suppose we initially have a high. Health, safety and technical notes. Repeat the experiment twice with different masses of water.
from chem.libretexts.org
See notes 5, 6 and 7 below for additional guidance. Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Repeat the experiment twice with different masses of water. Complete calculations to find the total mass of hot plus cold water. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Health, safety and technical notes. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result.
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Calorimetry Lab Ideas Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Read our standard health and safety guidance. Suppose we initially have a high. Complete calculations to find the total mass of hot plus cold water. See notes 5, 6 and 7 below for additional guidance. In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! Health, safety and technical notes. Repeat the experiment twice with different masses of water.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Ideas Suppose we initially have a high. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Read our standard health and safety guidance. Complete calculations to find the total mass of hot plus cold water. Health, safety and technical notes. In this project you will learn. Calorimetry Lab Ideas.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Lab Ideas Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Repeat the experiment twice with different masses of water. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Complete calculations. Calorimetry Lab Ideas.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Lab Ideas Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Read our standard health and safety guidance. Suppose we initially have a high. Complete calculations to find the total mass of hot. Calorimetry Lab Ideas.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Calorimetry Lab Ideas Complete calculations to find the total mass of hot plus cold water. Health, safety and technical notes. Repeat the experiment twice with different masses of water. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! Suppose we initially have a high. From the experiment setup and data collected,. Calorimetry Lab Ideas.
From www.youtube.com
Calorimetry Part I Lab Version) YouTube Calorimetry Lab Ideas Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! See notes 5, 6 and 7 below for additional guidance. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. From the experiment setup and. Calorimetry Lab Ideas.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Lab Ideas In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Read our standard health and safety guidance. Repeat the experiment twice with different masses of water. See notes 5, 6 and 7 below for additional guidance. Learn about calorimetry, make a bomb calorimeter, and experiment with. Calorimetry Lab Ideas.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Lab Ideas From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Health, safety and technical notes. Read our standard health and safety guidance. In this experiment you will heat a known mass of. Calorimetry Lab Ideas.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Calorimetry Lab Ideas Read our standard health and safety guidance. See notes 5, 6 and 7 below for additional guidance. Complete calculations to find the total mass of hot plus cold water. Suppose we initially have a high. Health, safety and technical notes. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Repeat. Calorimetry Lab Ideas.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Lab Ideas In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. See notes 5, 6 and 7 below for additional guidance. Health,. Calorimetry Lab Ideas.
From www.pinterest.com
Calorimeter Lab for General Science Physical science activities Calorimetry Lab Ideas Health, safety and technical notes. Read our standard health and safety guidance. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! See notes 5, 6 and 7 below for. Calorimetry Lab Ideas.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Ideas Repeat the experiment twice with different masses of water. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Health, safety and technical notes. Complete calculations to find the total. Calorimetry Lab Ideas.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Ideas See notes 5, 6 and 7 below for additional guidance. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Read our standard health and safety guidance. In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your. Calorimetry Lab Ideas.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Calorimetry Lab Ideas Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. See notes 5, 6 and 7 below for additional guidance. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Repeat the experiment twice with different masses of water. In this project. Calorimetry Lab Ideas.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Lab Ideas Repeat the experiment twice with different masses of water. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Complete calculations to find the total mass of hot plus cold water. Suppose we initially have a high. In this project you will learn a method for measuring how much chemical energy. Calorimetry Lab Ideas.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Lab Ideas In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. See notes 5, 6 and 7 below for additional guidance. Read our standard health and safety guidance. Complete calculations to find the total mass of hot plus cold water. Health, safety and technical notes. Suppose we. Calorimetry Lab Ideas.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Calorimetry Lab Ideas Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. Read our standard health and safety guidance. Before we practice calorimetry problems involving chemical. Calorimetry Lab Ideas.
From isabelladp.weebly.com
Calorimetry Lab Belle's Digital Portfolio Calorimetry Lab Ideas Read our standard health and safety guidance. Health, safety and technical notes. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. In this experiment you will heat a known mass of. Calorimetry Lab Ideas.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Ideas Repeat the experiment twice with different masses of water. Health, safety and technical notes. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. In this project. Calorimetry Lab Ideas.
From studylib.net
experiment 9 Thermochemistry Calorimetry Calorimetry Lab Ideas Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Calorimetry Lab Ideas.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Lab Ideas Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! Repeat the experiment twice with different masses of water. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Read our standard health and safety guidance. Before we practice calorimetry. Calorimetry Lab Ideas.
From www.youtube.com
Energy in Foods Calorimetry Lab YouTube Calorimetry Lab Ideas Health, safety and technical notes. Complete calculations to find the total mass of hot plus cold water. See notes 5, 6 and 7 below for additional guidance. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. From the experiment setup and data collected, students will. Calorimetry Lab Ideas.
From www.studocu.com
Lab02Calorimetry Lectures and activities LABORATORY ACTIVITY 02 Calorimetry Lab Ideas In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. See notes 5, 6 and 7 below for additional guidance. Read our standard health and safety guidance. Suppose we initially have a high. Health, safety and technical notes. From the experiment setup and data collected, students. Calorimetry Lab Ideas.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry Lab Ideas Complete calculations to find the total mass of hot plus cold water. In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Suppose we initially have. Calorimetry Lab Ideas.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Calorimetry Lab Ideas In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. See notes 5, 6 and 7 below for additional guidance. Suppose we initially have a high.. Calorimetry Lab Ideas.
From users.highland.edu
Calorimetry Calorimetry Lab Ideas Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Repeat the experiment twice with different masses of water. Read our standard health and safety guidance. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the. Calorimetry Lab Ideas.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Lab Ideas In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Repeat the experiment twice with different masses of water. Health, safety and technical notes. Learn about calorimetry,. Calorimetry Lab Ideas.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimetry Lab Ideas See notes 5, 6 and 7 below for additional guidance. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Repeat the experiment twice with different masses. Calorimetry Lab Ideas.
From www.pinterest.com
Put Some Energy Into It! Use a Calorimeter to Measure the Heat Capacity Calorimetry Lab Ideas Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. In this project you will learn a method for measuring how much chemical energy is stored in different types of. Calorimetry Lab Ideas.
From studylib.net
Lab 33 calorimetry lab Calorimetry Lab Ideas Complete calculations to find the total mass of hot plus cold water. In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea. Calorimetry Lab Ideas.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Lab Ideas Health, safety and technical notes. See notes 5, 6 and 7 below for additional guidance. In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it. Calorimetry Lab Ideas.
From www.youtube.com
Calorimetry Lab 2 YouTube Calorimetry Lab Ideas Suppose we initially have a high. Repeat the experiment twice with different masses of water. Learn about calorimetry, make a bomb calorimeter, and experiment with combusting different nuts to see which one produces the most energy! From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. See notes 5, 6 and. Calorimetry Lab Ideas.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Lab Ideas Repeat the experiment twice with different masses of water. Suppose we initially have a high. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. See notes 5, 6 and 7 below for additional guidance. In this project you will learn a method for measuring how. Calorimetry Lab Ideas.
From www.youtube.com
Calorimetry Experiment YouTube Calorimetry Lab Ideas Read our standard health and safety guidance. Health, safety and technical notes. Suppose we initially have a high. In this project you will learn a method for measuring how much chemical energy is stored in different types of food and express your result. Complete calculations to find the total mass of hot plus cold water. Learn about calorimetry, make a. Calorimetry Lab Ideas.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Calorimetry Lab Ideas Repeat the experiment twice with different masses of water. From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. Suppose we initially have a high. Complete calculations to find the total mass of hot plus cold water. In this experiment you will heat a known mass of a metal to a. Calorimetry Lab Ideas.
From studylib.net
Lab Calorimetry High School Science Help Calorimetry Lab Ideas From the experiment setup and data collected, students will have the evidence necessary to construct a model of heat. See notes 5, 6 and 7 below for additional guidance. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Complete calculations to find the total mass of hot plus cold water.. Calorimetry Lab Ideas.