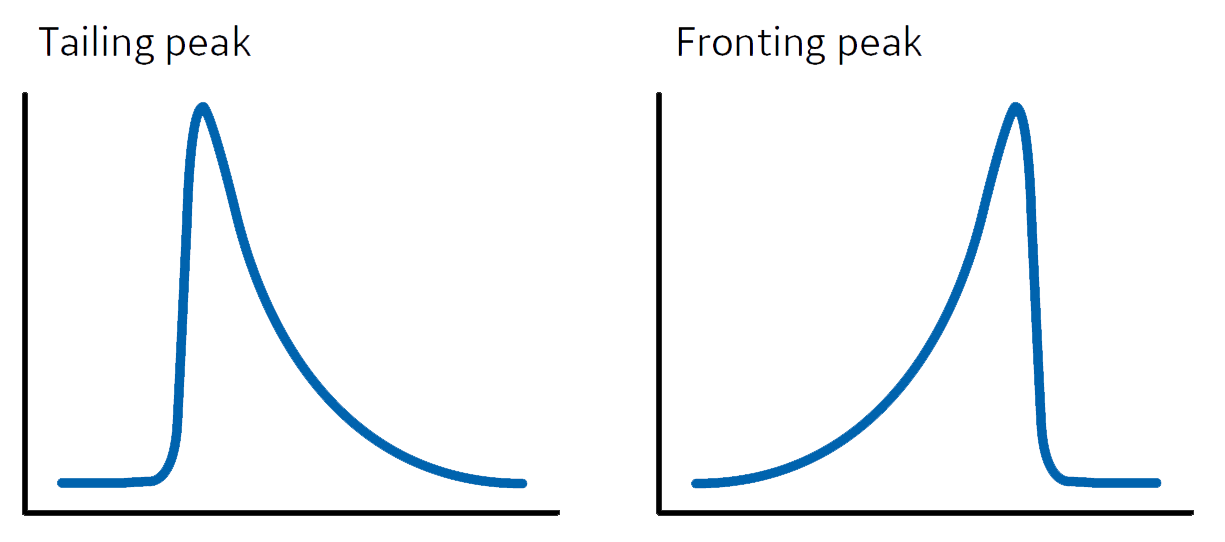
Chromatography Peaks . The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the purity of the separated proteins. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. Each peak corresponds to a specific elution time, measured from injection to peak maximum. In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Figure 12.2.9 b, which is an example of peak fronting most often is the result of overloading the column with sample. By comparing retention times [tr] with reference standards in the same chromatographic. The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter.
from www.cytivalifesciences.com
In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. Each peak corresponds to a specific elution time, measured from injection to peak maximum. Figure 12.2.9 b, which is an example of peak fronting most often is the result of overloading the column with sample. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the purity of the separated proteins. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used.
How to fix asymmetrical chromatography peak Cytiva
Chromatography Peaks Figure 12.2.9 b, which is an example of peak fronting most often is the result of overloading the column with sample. Figure 12.2.9 b, which is an example of peak fronting most often is the result of overloading the column with sample. In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. By comparing retention times [tr] with reference standards in the same chromatographic. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the purity of the separated proteins. The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. Each peak corresponds to a specific elution time, measured from injection to peak maximum. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used.
From www.cytivalifesciences.com
How to fix asymmetrical chromatography peak Cytiva Chromatography Peaks By comparing retention times [tr] with reference standards in the same chromatographic. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. Each peak corresponds to a specific elution time, measured from injection to peak maximum. Figure 12.2.9 b, which is an example of peak fronting most often is. Chromatography Peaks.
From www.researchgate.net
(PDF) Width Based Quantitation of Chromatographic Peaks. Principles and Chromatography Peaks If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. By comparing retention times [tr] with reference standards in the same chromatographic. In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal. Chromatography Peaks.
From www.researchgate.net
Full chromatograph of one reference gas measurement. First three peaks Chromatography Peaks By comparing retention times [tr] with reference standards in the same chromatographic. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more. Chromatography Peaks.
From www.barts-blog.net
arepeaksplittingandpeakfrontingeffectskeepingyouupatnight Chromatography Peaks Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the purity of the separated proteins. Chromatography is a method by which a mixture is separated by distributing its components between two. Chromatography Peaks.
From www.researchgate.net
Profiles of chromatographic peaks. The chromatograms of M, M + 1, and Chromatography Peaks But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. The chromatographic peak in figure 12.2.9 a is an. Chromatography Peaks.
From www.youtube.com
Introduction to Chromatography 6 Retention Time YouTube Chromatography Peaks The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. By comparing retention times [tr] with reference standards in the same chromatographic. Here are some basic. Chromatography Peaks.
From www.researchgate.net
Analysis of sizeexclusion chromatography profiles. Gaussian peaks fit Chromatography Peaks The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. Here are some basic definitions of all the terms. Chromatography Peaks.
From www.slideshare.net
Integration of chromatographic peaks Chromatography Peaks The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. Chromatography. Chromatography Peaks.
From chem.libretexts.org
12.2 General Theory of Column Chromatography Chemistry LibreTexts Chromatography Peaks By comparing retention times [tr] with reference standards in the same chromatographic. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. In practice, one can obtain peaks that tail, front,. Chromatography Peaks.
From aistsw.com
Chromatography Peak Modeling (Tutorial) Chromatography Peaks Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. Figure 12.2.9 b, which is an example of peak. Chromatography Peaks.
From lab-training.com
chromatographic calculations Chromatography Peaks The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. Figure 12.2.9 b, which is an example of peak fronting most often is the result of. Chromatography Peaks.
From www.youtube.com
Chromatography Peak Detection and Integration for HPLC & GC analysis Chromatography Peaks Chromatography is a method by which a mixture is separated by distributing its components between two phases. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is.. Chromatography Peaks.
From www.semanticscholar.org
Figure 1 from Integration Errors in Chromatographic Analysis, Part I Chromatography Peaks But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. By comparing retention times [tr] with reference standards in the same chromatographic. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a. Chromatography Peaks.
From www.researchgate.net
Examples of the shoulder peak, indicated by blue arrows, in the Chromatography Peaks In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. Each peak corresponds to a specific elution time, measured from injection. Chromatography Peaks.
From www.lachmanconsultants.com
Extraneous Chromatographic Peaks What’s a Lab to Do? Lachman Chromatography Peaks The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. Chromatography is a method by which a mixture is. Chromatography Peaks.
From www.thoughtco.com
Gas Chromatography What It Is and How It Works Chromatography Peaks By comparing retention times [tr] with reference standards in the same chromatographic. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Figure 12.2.9 b, which is. Chromatography Peaks.
From www.researchgate.net
Representative chromatogram identifying the peaks isolated in this Chromatography Peaks If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. Each peak corresponds to a specific elution time, measured. Chromatography Peaks.
From instrumentationtools.com
Online Gas Chromatograph Principle InstrumentationTools Chromatography Peaks If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. Chromatography is a method by which a mixture is separated by. Chromatography Peaks.
From www.degruyter.com
Peaks and more Chromatography Peaks Chromatography is a method by which a mixture is separated by distributing its components between two phases. Figure 12.2.9 b, which is an example of peak fronting most often is the result of overloading the column with sample. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. The. Chromatography Peaks.
From www.researchgate.net
GCFID data formats. A. Three hypothetical chromatograms are shown Chromatography Peaks By comparing retention times [tr] with reference standards in the same chromatographic. The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as. Chromatography Peaks.
From chem.libretexts.org
12.2 General Theory of Column Chromatography Chemistry LibreTexts Chromatography Peaks Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the purity of the separated proteins. By comparing retention times [tr] with reference standards in the same chromatographic. The stationary phase remains. Chromatography Peaks.
From www.researchgate.net
HPLC chromatogram of a standard mixture at 20 mg L⁻¹. Peak Chromatography Peaks But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. By comparing retention. Chromatography Peaks.
From lab-training.com
Gas Chromatography Principles, Types and Working Chromatography Peaks The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. Figure 12.2.9 b, which is an example of peak fronting most often is the result of. Chromatography Peaks.
From www.researchgate.net
HPLC chromatography peaks from the honeydew samples collected from Chromatography Peaks Figure 12.2.9 b, which is an example of peak fronting most often is the result of overloading the column with sample. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such. Chromatography Peaks.
From www.barts-blog.net
The perfect peak shape Five solutions to peak tailing problems Chromatography Peaks Chromatography is a method by which a mixture is separated by distributing its components between two phases. If you want to master the art of interpreting a chromatogram, you first need to know exactly what a chromatogram is. By comparing retention times [tr] with reference standards in the same chromatographic. But before moving on to that, let’s first take a. Chromatography Peaks.
From chem.libretexts.org
12.4 Gas Chromatography Chemistry LibreTexts Chromatography Peaks Each peak corresponds to a specific elution time, measured from injection to peak maximum. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. By comparing retention times [tr] with reference. Chromatography Peaks.
From chem.libretexts.org
12.2 General Theory of Column Chromatography Chemistry LibreTexts Chromatography Peaks In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. Chromatography is a. Chromatography Peaks.
From chem.libretexts.org
12.4 Gas Chromatography Chemistry LibreTexts Chromatography Peaks The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. In practice, one can obtain peaks that tail, front,. Chromatography Peaks.
From www.researchgate.net
11 High resolution ion exchange chromatography of the peaks obtained Chromatography Peaks The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. But before moving on to that, let’s first take a look at chromatography, its advantages, types, and other details that will further help in the understanding of a chromatogram. Take. Chromatography Peaks.
From chemwiki.ucdavis.edu
High performance liquid chromatography Chemwiki Chromatography Peaks In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. Chromatography is a method by which a mixture is separated by distributing its components between two phases. If you want to master the art of interpreting a chromatogram, you first need to. Chromatography Peaks.
From chem.libretexts.org
12.2 General Theory of Column Chromatography Chemistry LibreTexts Chromatography Peaks Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. Each peak corresponds to a specific elution time, measured from injection to peak maximum. Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks. Chromatography Peaks.
From peakperformanceanalytical.com
Process GC Problems? Look at Your Chromatogram! Peak Performance Chromatography Peaks Each peak corresponds to a specific elution time, measured from injection to peak maximum. By comparing retention times [tr] with reference standards in the same chromatographic. Take a look at our guide to understanding the different peaks on a chromatogram, and to troubleshooting any problems you might encounter. Chromatography is a method by which a mixture is separated by distributing. Chromatography Peaks.
From www.barts-blog.net
How to choose a stationary phase, optimize selectivity and get better Chromatography Peaks Figure 12.2.9 b, which is an example of peak fronting most often is the result of overloading the column with sample. Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the. Chromatography Peaks.
From www.researchgate.net
How to calculate components of overlapping peaks in chromatogram? Chromatography Peaks In practice, one can obtain peaks that tail, front, or concurrently front and tail for reasons such as column packing issues, chemical and kinetic effects, and suboptimal high. By comparing retention times [tr] with reference standards in the same chromatographic. The chromatographic peak in figure 12.2.9 a is an example of peak tailing, which occurs when some sites on the. Chromatography Peaks.
From lab-training.com
chromatographic calculations Chromatography Peaks Here are some basic definitions of all the terms related to peaks, what you can determine from the fplc peaks, and what the peaks can tell you about the quality of column packing, the efficiency of separation, and the purity of the separated proteins. But before moving on to that, let’s first take a look at chromatography, its advantages, types,. Chromatography Peaks.