Potassium Hydroxide And Zinc Chloride Precipitate . Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. To predict the product of a. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Because both components of each. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Updated on october 09, 2019. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Enter an equation of an ionic chemical equation and press the balance button. Here’s the best way to solve it. This guide will show how to. The balanced equation will be calculated along with the. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate.
from wisc.pb.unizin.org
The balanced equation will be calculated along with the. Because both components of each. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. This guide will show how to. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Updated on october 09, 2019. Enter an equation of an ionic chemical equation and press the balance button. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. To predict the product of a.
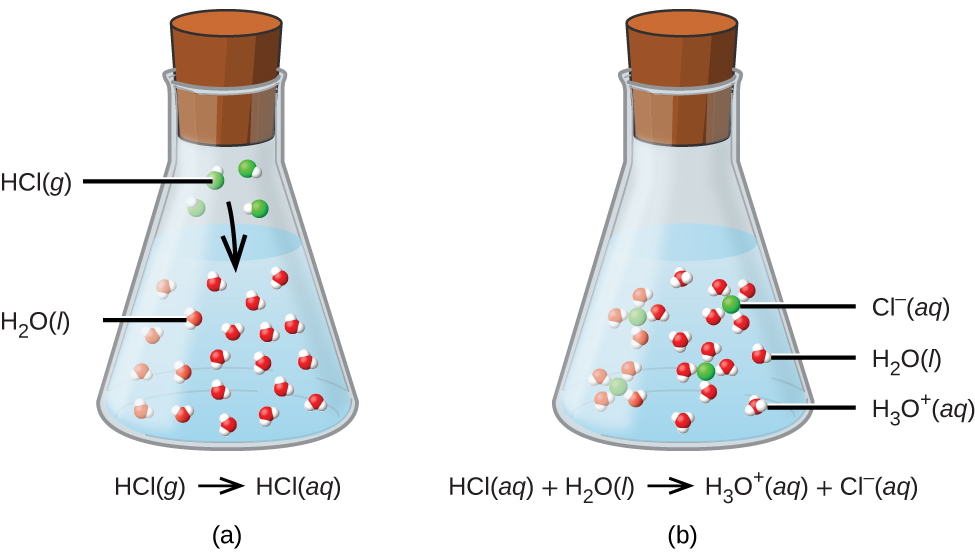
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW
Potassium Hydroxide And Zinc Chloride Precipitate When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Because both components of each. Enter an equation of an ionic chemical equation and press the balance button. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. This guide will show how to. To predict the product of a. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Updated on october 09, 2019. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Here’s the best way to solve it. The balanced equation will be calculated along with the. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Potassium Hydroxide And Zinc Chloride Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. The balanced equation will be. Potassium Hydroxide And Zinc Chloride Precipitate.
From yixinchemical.allweyes.com
zinc hydroxide precipitate Yixin Potassium Hydroxide And Zinc Chloride Precipitate To predict the product of a. This guide will show how to. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Here’s the best way to solve it. Updated on october 09, 2019. The balanced equation will. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.researchgate.net
Zinc hydroxide (ZH) precipitation during the titration of 0.1 m NaOH Potassium Hydroxide And Zinc Chloride Precipitate Updated on october 09, 2019. The balanced equation will be calculated along with the. Because both components of each. To predict the product of a. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms. Potassium Hydroxide And Zinc Chloride Precipitate.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Potassium Hydroxide And Zinc Chloride Precipitate When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? This guide will show how to. Here’s the best way to solve it. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. The balanced equation will be calculated along with the. Updated on october 09,. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Potassium Hydroxide And Zinc Chloride Precipitate Here’s the best way to solve it. Enter an equation of an ionic chemical equation and press the balance button. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. When a solution. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.slideserve.com
PPT Precipitation Reactions (Double Replacement Reactions) PowerPoint Potassium Hydroxide And Zinc Chloride Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. To predict the product of a. This guide will show how to. Because both components of each. Enter an equation of an ionic chemical equation and press the balance button. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.sciencephoto.com
Zinc Hydroxide Precipitate Stock Image C027/9442 Science Photo Potassium Hydroxide And Zinc Chloride Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Updated on october 09, 2019. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed.. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.numerade.com
SOLVED Upon mixing, which of the following solutions will produce ONE Potassium Hydroxide And Zinc Chloride Precipitate Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Because both components of each. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. To. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.numerade.com
SOLVED When aqueous solutions of potassium phosphate and zinc(II Potassium Hydroxide And Zinc Chloride Precipitate Updated on october 09, 2019. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? To predict the product of a. This guide will show how to. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. A substance will precipitate. Potassium Hydroxide And Zinc Chloride Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Potassium Hydroxide And Zinc Chloride Precipitate Updated on october 09, 2019. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate.. Potassium Hydroxide And Zinc Chloride Precipitate.
From woelen.homescience.net
Science made alive Chemistry/Solutions Potassium Hydroxide And Zinc Chloride Precipitate Because both components of each. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Updated on october 09, 2019. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. The balanced equation will be calculated along with the. To predict the product of a. Here’s. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Potassium Hydroxide And Zinc Chloride Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. This guide will show how to. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Here’s. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Calcium chloride (CaCl2) form a Potassium Hydroxide And Zinc Chloride Precipitate Here’s the best way to solve it. This guide will show how to. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? In a precipitation reaction, a subclass of exchange reactions, an insoluble material. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.numerade.com
SOLVED Write an unbalanced equation to represent each of the following Potassium Hydroxide And Zinc Chloride Precipitate In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Updated. Potassium Hydroxide And Zinc Chloride Precipitate.
From questions.kunduz.com
Complete the table below by deciding whet... Physical Chemistry Potassium Hydroxide And Zinc Chloride Precipitate This guide will show how to. Because both components of each. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Updated on october 09, 2019. Enter an equation of an ionic chemical equation and press the balance. Potassium Hydroxide And Zinc Chloride Precipitate.
From yixinchemical.allweyes.com
zinc hydroxide precipitate Yixin Potassium Hydroxide And Zinc Chloride Precipitate Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. To predict the product of a. Updated on october 09, 2019. This guide will show how to. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Because both components of each. In. Potassium Hydroxide And Zinc Chloride Precipitate.
From fineartamerica.com
Zinc Hydroxide Precipitate Photograph by Andrew Lambert Photography Potassium Hydroxide And Zinc Chloride Precipitate A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? When two aqueous solutions of ionic. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Potassium Hydroxide And Zinc Chloride Precipitate Updated on october 09, 2019. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Here’s the best way to solve it. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. The balanced equation will be calculated along with the. In a precipitation reaction, a subclass of. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Potassium Hydroxide And Zinc Chloride Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? This guide will show how to. Enter an equation of an ionic chemical equation and press the balance button. In a precipitation reaction, a subclass of exchange reactions,. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Potassium Hydroxide And Zinc Chloride Precipitate When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. The balanced equation will be calculated along with the. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Here’s. Potassium Hydroxide And Zinc Chloride Precipitate.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Potassium Hydroxide And Zinc Chloride Precipitate Here’s the best way to solve it. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. A precipitation reaction is a reaction that yields an insoluble product—a. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.slideserve.com
PPT Na 3 PO 4 + 3 KOH â 3 NaOH + K 3 PO 4 Double Displacement Potassium Hydroxide And Zinc Chloride Precipitate To predict the product of a. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Updated on october 09, 2019. This guide will show how to. When a solution of potassium hydroxide. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.toppr.com
Given below are word equations. Convert them into chemical equations Potassium Hydroxide And Zinc Chloride Precipitate In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. The balanced equation will be calculated along with the. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Here’s the best way to solve it. Enter an equation of an. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.numerade.com
Problem 5. From qualitative analysis, a solid, colorless compound was Potassium Hydroxide And Zinc Chloride Precipitate Updated on october 09, 2019. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Potassium Hydroxide And Zinc Chloride Precipitate Because both components of each. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. To predict the product of a. A precipitation reaction is a reaction that yields an insoluble product—a. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved Predicting precipitation Complete the table below by Potassium Hydroxide And Zinc Chloride Precipitate Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Here’s the best way to solve it. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Enter an equation of an ionic chemical equation and press the. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.researchgate.net
Comparison of Zn content in the precipitate after precipitation with Potassium Hydroxide And Zinc Chloride Precipitate Because both components of each. The balanced equation will be calculated along with the. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Here’s the best way to solve it. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Updated on october 09, 2019. Precipitation reactions. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.numerade.com
SOLVEDsolid zinc hydrochloric acid hydrogen gas aqueous zinc chloride Potassium Hydroxide And Zinc Chloride Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? Updated on october 09, 2019. Here’s the best way to solve it. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Zinc sulfate (ZnSO4) form a Potassium Hydroxide And Zinc Chloride Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Updated on october 09, 2019. The balanced equation will be calculated along with the. Here’s the best way to solve it. A. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.slideshare.net
Precipitation react2 Potassium Hydroxide And Zinc Chloride Precipitate Updated on october 09, 2019. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. This guide will show how to. Because both components of each. Precipitation reactions are a subclass. Potassium Hydroxide And Zinc Chloride Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Potassium Hydroxide And Zinc Chloride Precipitate This guide will show how to. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. The balanced equation will be calculated along with the. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? In a precipitation reaction, a subclass of exchange reactions, an. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved Enter an equation for the precipitation reaction that Potassium Hydroxide And Zinc Chloride Precipitate Here’s the best way to solve it. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Updated on october 09, 2019. Because both components of each. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. When a solution of potassium hydroxide. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Potassium Hydroxide And Zinc Chloride Precipitate Because both components of each. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. This. Potassium Hydroxide And Zinc Chloride Precipitate.
From questions.kunduz.com
Complete the table below by deciding whe... Chemistry Potassium Hydroxide And Zinc Chloride Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. When a solution of potassium hydroxide and zinc chloride are mixed, what precipitate if any is formed? A substance will precipitate when solution conditions are. Potassium Hydroxide And Zinc Chloride Precipitate.
From www.pinterest.com
Precipitation Reaction a reaction that results in the formation of an Potassium Hydroxide And Zinc Chloride Precipitate This guide will show how to. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Here’s the best way to solve it. A precipitation reaction is a. Potassium Hydroxide And Zinc Chloride Precipitate.