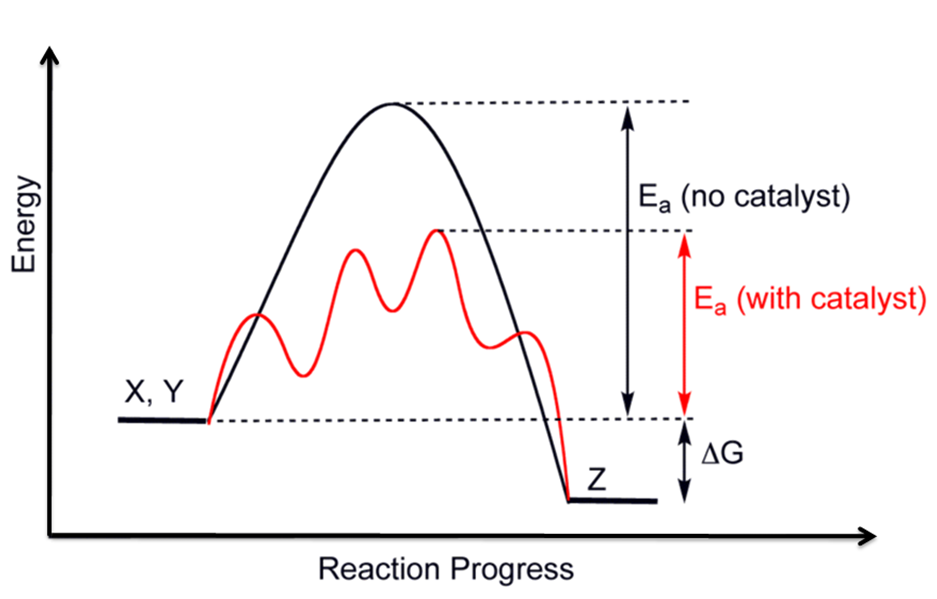
Catalyst Reaction Graph . Catalysts function by providing an alternate reaction mechanism that has a lower. The two reaction diagrams here represent the same reaction: The graph below shows the energy of a reaction both with and without a catalyst present. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Reaction diagrams for catalyzed reactions. The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. The only effect of the catalyst is to lower the activation energy of the. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Reaction diagrams for catalyzed reactions. One without a catalyst and one with a.
from diagramlibraryoily.z5.web.core.windows.net
Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. This page explains how adding a catalyst affects the rate of a reaction. One without a catalyst and one with a. The graph below shows the energy of a reaction both with and without a catalyst present. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The two reaction diagrams here represent the same reaction: It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts function by providing an alternate reaction mechanism that has a lower.
Energy Diagram Catalyst
Catalyst Reaction Graph One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The two reaction diagrams here represent the same reaction: This page describes and explains the way that adding a catalyst affects the rate of a reaction. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The only effect of the catalyst is to lower the activation energy of the. This page explains how adding a catalyst affects the rate of a reaction. One without a catalyst and one with a. Reaction diagrams for catalyzed reactions. The graph below shows the energy of a reaction both with and without a catalyst present. Catalysts function by providing an alternate reaction mechanism that has a lower.
From 2012books.lardbucket.org
Catalysis Catalyst Reaction Graph Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. Reaction diagrams for catalyzed reactions. The graph below shows the energy of a reaction both with and without a catalyst present. One without a catalyst and one with a. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The only. Catalyst Reaction Graph.
From courses.lumenlearning.com
Catalysis Chemistry for Majors Catalyst Reaction Graph The graph below shows the energy of a reaction both with and without a catalyst present. The two reaction diagrams here represent the same reaction: The two reaction diagrams here represent the same reaction: The only effect of the catalyst is to lower the activation energy of the. Reaction diagrams for catalyzed reactions. This page describes and explains the way. Catalyst Reaction Graph.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Reaction Graph This page describes and explains the way that adding a catalyst affects the rate of a reaction. The two reaction diagrams here represent the same reaction: This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Reaction diagrams for catalyzed reactions. The. Catalyst Reaction Graph.
From diagramlibraryoily.z5.web.core.windows.net
Energy Diagram Catalyst Catalyst Reaction Graph The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. Catalysts function by providing an alternate reaction mechanism that has a lower. The two reaction diagrams here represent the same reaction: Reaction diagrams for catalyzed reactions. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The graph below shows the. Catalyst Reaction Graph.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Reaction Graph The graph below shows the energy of a reaction both with and without a catalyst present. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Reaction diagrams for catalyzed reactions. The only effect of the catalyst is to lower. Catalyst Reaction Graph.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Reaction Graph It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts function by providing an alternate reaction mechanism that has a lower. The two reaction diagrams here represent the same reaction: The only effect of the catalyst is to lower the activation energy of the. Reaction diagrams for catalyzed reactions. This page explains how. Catalyst Reaction Graph.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalyst Reaction Graph Catalysts function by providing an alternate reaction mechanism that has a lower. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. One without a catalyst and one with a. The graph below shows the energy of a reaction both with and without a catalyst. Catalyst Reaction Graph.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Reaction Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. One without a catalyst and one with a. One without a catalyst and one with a. The only effect of the catalyst is to lower the activation energy of the. This page explains how adding a catalyst affects the rate of a reaction. Reaction diagrams for catalyzed reactions.. Catalyst Reaction Graph.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Catalyst Reaction Graph It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts function by providing an alternate reaction mechanism that has a lower. Reaction diagrams for catalyzed reactions. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The only effect of the catalyst is to lower the. Catalyst Reaction Graph.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Reaction Graph One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: It assumes that you are already familiar with basic ideas about the collision theory of reaction. Reaction diagrams for catalyzed reactions. The graph below shows the energy of a reaction both with and without a catalyst present. Catalysts function by providing an alternate. Catalyst Reaction Graph.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Catalyst Reaction Graph Catalysts function by providing an alternate reaction mechanism that has a lower. The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The only effect of the catalyst is to lower the. Catalyst Reaction Graph.
From www.catalystseurope.org
What are catalysts? Catalyst Reaction Graph One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The only effect of the catalyst is to lower the. Catalyst Reaction Graph.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Catalyst Reaction Graph One without a catalyst and one with a. The graph below shows the energy of a reaction both with and without a catalyst present. The only effect of the catalyst is to lower the activation energy of the. Catalysts function by providing an alternate reaction mechanism that has a lower. This page describes and explains the way that adding a. Catalyst Reaction Graph.
From www.thesciencehive.co.uk
Reaction Rates* — the science sauce Catalyst Reaction Graph Catalysts function by providing an alternate reaction mechanism that has a lower. The two reaction diagrams here represent the same reaction: The graph below shows the energy of a reaction both with and without a catalyst present. One without a catalyst and one with a. The only effect of the catalyst is to lower the activation energy of the. It. Catalyst Reaction Graph.
From wiringzoophiles.z21.web.core.windows.net
Energy Diagrams For Two Reactions Are Shown Catalyst Reaction Graph The graph below shows the energy of a reaction both with and without a catalyst present. One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts function by providing an alternate reaction mechanism that has a lower. This graph compares the reaction coordinates for. Catalyst Reaction Graph.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Reaction Graph One without a catalyst and one with a. One without a catalyst and one with a. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The only effect of the catalyst is to lower the activation energy of the. Reaction diagrams for catalyzed reactions. The two reaction diagrams here represent the same reaction:. Catalyst Reaction Graph.
From userwiringmcknight.z5.web.core.windows.net
Reaction Profile Diagram Catalyst Reaction Graph This page explains how adding a catalyst affects the rate of a reaction. The only effect of the catalyst is to lower the activation energy of the. The graph below shows the energy of a reaction both with and without a catalyst present. This page describes and explains the way that adding a catalyst affects the rate of a reaction.. Catalyst Reaction Graph.
From nonsibihighschool.org
Section 184 Catalysis Catalyst Reaction Graph One without a catalyst and one with a. The only effect of the catalyst is to lower the activation energy of the. The two reaction diagrams here represent the same reaction: This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a. Catalyst Reaction Graph.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Reaction Graph Catalysts function by providing an alternate reaction mechanism that has a lower. One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: This page explains how adding a catalyst affects the rate of a reaction. The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. Reaction. Catalyst Reaction Graph.
From byjus.com
Choose the graph which correctly represents the reaction path when a Catalyst Reaction Graph The two reaction diagrams here represent the same reaction: It assumes that you are already familiar with basic ideas about the collision theory of reaction. The graph below shows the energy of a reaction both with and without a catalyst present. The two reaction diagrams here represent the same reaction: This page explains how adding a catalyst affects the rate. Catalyst Reaction Graph.
From diagramlisthavens.z21.web.core.windows.net
Reaction Coordinate Diagram With Catalyst Catalyst Reaction Graph The graph below shows the energy of a reaction both with and without a catalyst present. The two reaction diagrams here represent the same reaction: Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are. Catalyst Reaction Graph.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate Catalyst Reaction Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The graph below shows the energy of a reaction both with and without a catalyst present. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The two reaction diagrams here represent the same reaction: This page describes and explains the way. Catalyst Reaction Graph.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalyst Reaction Graph The two reaction diagrams here represent the same reaction: Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. This page explains how adding a catalyst affects the rate of a reaction. One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction.. Catalyst Reaction Graph.
From usermanualwhelming.z21.web.core.windows.net
Reaction Profile Diagram With Catalyst Catalyst Reaction Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts function by providing an alternate reaction mechanism that has a lower. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains. Catalyst Reaction Graph.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Reaction Graph The graph below shows the energy of a reaction both with and without a catalyst present. The only effect of the catalyst is to lower the activation energy of the. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts function by providing an alternate reaction mechanism that has a lower. Reaction. Catalyst Reaction Graph.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Reaction Graph Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. This page explains how adding a catalyst affects the rate of a reaction. One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts function by providing an alternate reaction mechanism that. Catalyst Reaction Graph.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Reaction Graph It assumes that you are already familiar with basic ideas about the collision theory of reaction. The only effect of the catalyst is to lower the activation energy of the. The two reaction diagrams here represent the same reaction: Reaction diagrams for catalyzed reactions. The two reaction diagrams here represent the same reaction: One without a catalyst and one with. Catalyst Reaction Graph.
From www.pnnl.gov
Catalysis PNNL Catalyst Reaction Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. One without a catalyst and one with a. Catalysts function by providing an alternate reaction mechanism that has a lower. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The two reaction diagrams here represent the same reaction: The only. Catalyst Reaction Graph.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Reaction Graph The two reaction diagrams here represent the same reaction: The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This page explains how adding. Catalyst Reaction Graph.
From guidefixcasiranilv.z4.web.core.windows.net
How To Read Energy Diagrams Chemistry Catalyst Reaction Graph The only effect of the catalyst is to lower the activation energy of the. Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction.. Catalyst Reaction Graph.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Reaction Graph This page explains how adding a catalyst affects the rate of a reaction. The only effect of the catalyst is to lower the activation energy of the. Catalysts function by providing an alternate reaction mechanism that has a lower. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The two reaction diagrams here. Catalyst Reaction Graph.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Reaction Graph This page describes and explains the way that adding a catalyst affects the rate of a reaction. One without a catalyst and one with a. One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page. Catalyst Reaction Graph.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Reaction Graph The only effect of the catalyst is to lower the activation energy of the. One without a catalyst and one with a. Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. The graph below shows the energy of a reaction both with and without a catalyst present. This page explains how adding a catalyst affects the. Catalyst Reaction Graph.
From www.varsitytutors.com
Reaction Coordinate Diagrams College Chemistry Catalyst Reaction Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Reaction diagrams for catalyzed reactions. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The only effect of the catalyst is to lower the activation energy of the. One without a catalyst and one with a. Catalysts function by providing an. Catalyst Reaction Graph.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Reaction Graph Catalysts function by providing an alternate reaction mechanism that has a lower. Reaction diagrams for catalyzed reactions. The graph below shows the energy of a reaction both with and without a catalyst present. The only effect of the catalyst is to lower the activation energy of the. Reaction diagrams for catalyzed reactions. It assumes that you are already familiar with. Catalyst Reaction Graph.