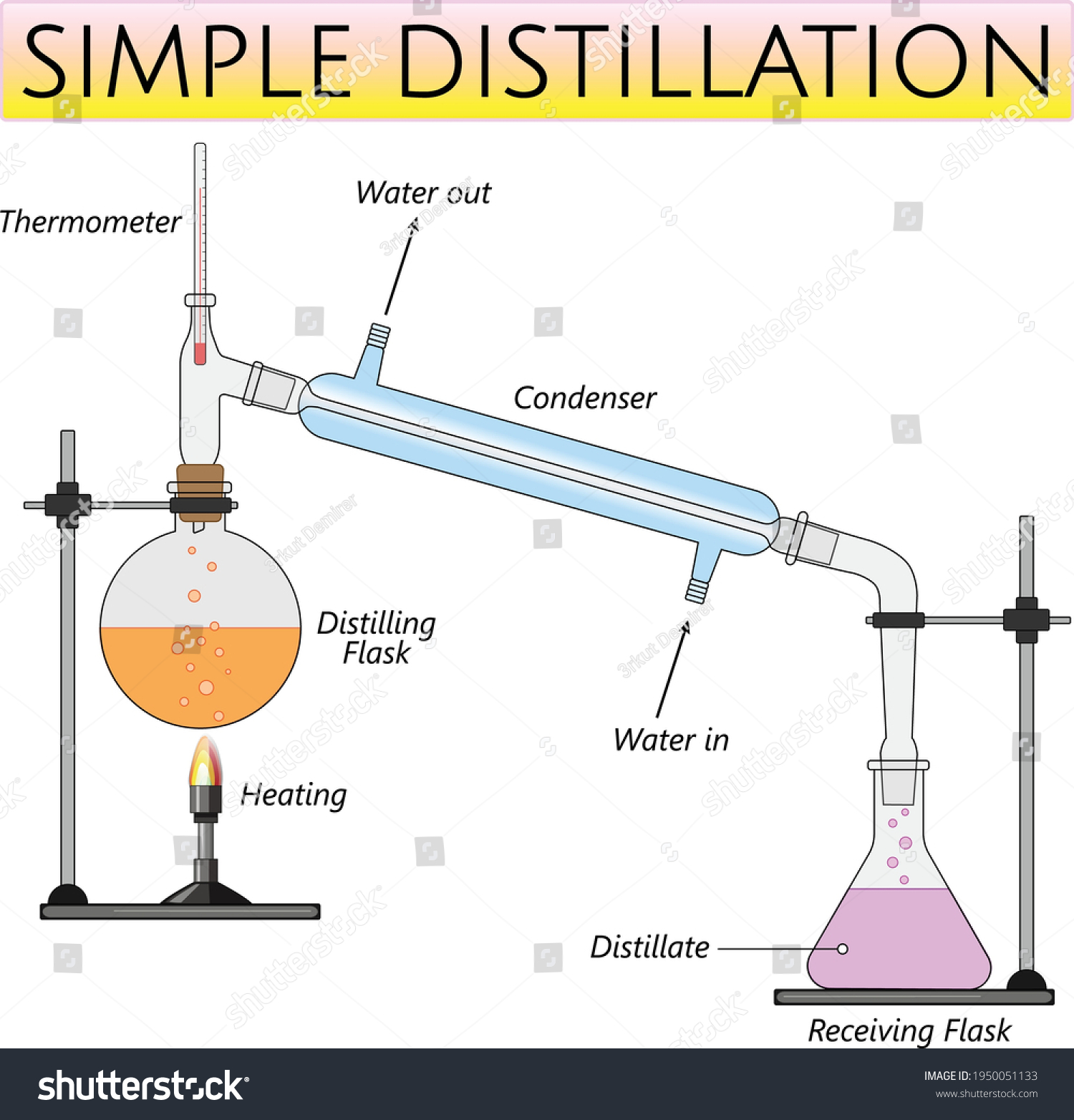
Distillation Experiment Class 9 . There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Steps for separation of ammonium chloride: Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a separation technique used to remove a solvent from a mixture and keep it. In this method, the mixture to be separated is heated and then cooled using a water condenser. Take the mixture of sand, ammonium chloride and common salt in a china dish and. What is a simple distillation experiment? Investigate the separation of water from a coloured solution using this simple distillation video, including. Aim to separate the components of a mixture of sand, common salt. Learn more in this ks3 chemistry guide from bitesize. Simple distillation separates components from their liquid mixtures based on their boiling point differences.
from www.shutterstock.com
Distillation is a separation technique used to remove a solvent from a mixture and keep it. Aim to separate the components of a mixture of sand, common salt. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Learn more in this ks3 chemistry guide from bitesize. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Investigate the separation of water from a coloured solution using this simple distillation video, including. Steps for separation of ammonium chloride: Simple distillation separates components from their liquid mixtures based on their boiling point differences. In this method, the mixture to be separated is heated and then cooled using a water condenser.
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous
Distillation Experiment Class 9 Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Aim to separate the components of a mixture of sand, common salt. Simple distillation separates components from their liquid mixtures based on their boiling point differences. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. What is a simple distillation experiment? Investigate the separation of water from a coloured solution using this simple distillation video, including. Distillation is a separation technique used to remove a solvent from a mixture and keep it. In this method, the mixture to be separated is heated and then cooled using a water condenser. Learn more in this ks3 chemistry guide from bitesize. Steps for separation of ammonium chloride: Take the mixture of sand, ammonium chloride and common salt in a china dish and.
From zamcopter.web.fc2.com
SIMPLE DISTILLATION Distillation Experiment Class 9 Steps for separation of ammonium chloride: In this method, the mixture to be separated is heated and then cooled using a water condenser. What is a simple distillation experiment? Learn more in this ks3 chemistry guide from bitesize. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Distillation refers to the selective boiling and subsequent condensation. Distillation Experiment Class 9.
From www.studocu.com
Simple distillation experiment report Min kyeong Sin March / 27 / Distillation Experiment Class 9 Take the mixture of sand, ammonium chloride and common salt in a china dish and. Investigate the separation of water from a coloured solution using this simple distillation video, including. Learn more in this ks3 chemistry guide from bitesize. Aim to separate the components of a mixture of sand, common salt. Distillation is a separation technique used to remove a. Distillation Experiment Class 9.
From www.turbosquid.com
Steam distillation laboratory kit 3D model TurboSquid 1458571 Distillation Experiment Class 9 Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Learn more in this ks3 chemistry guide from bitesize. Investigate the separation of water from a coloured solution using this simple distillation video, including. In this method, the mixture to be separated is heated and then cooled using a water condenser. Steps for separation. Distillation Experiment Class 9.
From www.youtube.com
Distillation Fraction Distillation Science Class 9th YouTube Distillation Experiment Class 9 In this method, the mixture to be separated is heated and then cooled using a water condenser. Simple distillation separates components from their liquid mixtures based on their boiling point differences. Distillation is a separation technique used to remove a solvent from a mixture and keep it. What is a simple distillation experiment? There are different ways to separate mixtures,. Distillation Experiment Class 9.
From www.yaclass.in
Simple distillation — lesson. Science CBSE, Class 9. Distillation Experiment Class 9 Learn more in this ks3 chemistry guide from bitesize. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Investigate the separation of water from a coloured solution using this simple distillation video, including. Aim to separate the components of a mixture of sand, common salt. Distillation is a separation technique used to remove a. Distillation Experiment Class 9.
From kolblabs.com
Separation of two miscible liquids by distillation in Is Matter Pure Distillation Experiment Class 9 Distillation is a separation technique used to remove a solvent from a mixture and keep it. In this method, the mixture to be separated is heated and then cooled using a water condenser. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Investigate the separation of water from a coloured solution using this simple. Distillation Experiment Class 9.
From mavink.com
Carte Mentale La Distillation Distillation Experiment Class 9 Aim to separate the components of a mixture of sand, common salt. Learn more in this ks3 chemistry guide from bitesize. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Simple distillation separates components from their liquid mixtures based on their boiling point differences. Distillation is a separation technique used to remove a solvent. Distillation Experiment Class 9.
From mavink.com
Fractional Distillation Diagram Class 8 Distillation Experiment Class 9 Investigate the separation of water from a coloured solution using this simple distillation video, including. Steps for separation of ammonium chloride: Distillation is a separation technique used to remove a solvent from a mixture and keep it. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. What is a simple distillation experiment? Distillation refers to the. Distillation Experiment Class 9.
From www.chemicalslearning.com
Simple Distillation Process Differential Distillation Process Distillation Experiment Class 9 What is a simple distillation experiment? There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Steps for separation of ammonium chloride: Take the mixture of sand, ammonium chloride and common salt in a china dish and. Aim to separate the components of a mixture of sand, common salt. Simple distillation separates components from their liquid mixtures. Distillation Experiment Class 9.
From www.youtube.com
DISTILLATION OF WATER AND ETHANOL YouTube Distillation Experiment Class 9 Learn more in this ks3 chemistry guide from bitesize. Distillation is a separation technique used to remove a solvent from a mixture and keep it. What is a simple distillation experiment? Steps for separation of ammonium chloride: Simple distillation separates components from their liquid mixtures based on their boiling point differences. In this method, the mixture to be separated is. Distillation Experiment Class 9.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Experiment Class 9 Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. In this method, the mixture to be separated is heated and then cooled using a water condenser. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. Simple distillation. Distillation Experiment Class 9.
From www.studocu.com
Experiment 2 Simple distillation Experiment 2 Simple distillation Distillation Experiment Class 9 Investigate the separation of water from a coloured solution using this simple distillation video, including. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. What is a simple distillation experiment? Steps for separation of ammonium chloride: Take the mixture of sand, ammonium chloride and common salt in a china dish and. Learn more. Distillation Experiment Class 9.
From pharmacygyan.com
Fractional distillation Principle Construction Working etc.. Pharmacy Gyan Distillation Experiment Class 9 Aim to separate the components of a mixture of sand, common salt. What is a simple distillation experiment? Learn more in this ks3 chemistry guide from bitesize. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Simple. Distillation Experiment Class 9.
From medium.com
‘Ultrapure Water and Distilled Water — What is the Difference? by Distillation Experiment Class 9 Take the mixture of sand, ammonium chloride and common salt in a china dish and. Steps for separation of ammonium chloride: Investigate the separation of water from a coloured solution using this simple distillation video, including. Learn more in this ks3 chemistry guide from bitesize. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Distillation is. Distillation Experiment Class 9.
From www.youtube.com
Science 9 Chapter 2 Distillation, Fractional Distillation Distillation Experiment Class 9 What is a simple distillation experiment? Simple distillation separates components from their liquid mixtures based on their boiling point differences. Investigate the separation of water from a coloured solution using this simple distillation video, including. Steps for separation of ammonium chloride: Take the mixture of sand, ammonium chloride and common salt in a china dish and. Aim to separate the. Distillation Experiment Class 9.
From www.yaclass.in
Simple distillation — lesson. Science State Board, Class 9. Distillation Experiment Class 9 There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Aim to separate the components of a mixture of sand, common salt. In this method, the. Distillation Experiment Class 9.
From www.geeksforgeeks.org
Distillation Definition, Meaning, Principle, Types & Uses Distillation Experiment Class 9 Learn more in this ks3 chemistry guide from bitesize. Distillation is a separation technique used to remove a solvent from a mixture and keep it. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Simple distillation separates components from their liquid mixtures based on their boiling point differences. What is a simple distillation experiment? Distillation refers. Distillation Experiment Class 9.
From ar.inspiredpencil.com
Fractional Distillation Chemistry Distillation Experiment Class 9 Simple distillation separates components from their liquid mixtures based on their boiling point differences. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Take the mixture of sand, ammonium chloride and common salt in a china dish. Distillation Experiment Class 9.
From lugomoni.wixsite.com
Experiment distillation Distillation Experiment Class 9 Aim to separate the components of a mixture of sand, common salt. What is a simple distillation experiment? Distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. In this method, the mixture to be separated is heated and then cooled using a water condenser.. Distillation Experiment Class 9.
From otrabalhosocomecou.macae.rj.gov.br
einzig und allein Gut ausgebildete Wesentlich appareil de distillation Distillation Experiment Class 9 Take the mixture of sand, ammonium chloride and common salt in a china dish and. Aim to separate the components of a mixture of sand, common salt. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Simple distillation separates components from their liquid mixtures based on their boiling point differences. In this method, the mixture to. Distillation Experiment Class 9.
From www.shutterstock.com
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous Distillation Experiment Class 9 There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Learn more in this ks3 chemistry guide from bitesize. In this method, the mixture to be separated is heated and then cooled using a water condenser. Aim to separate the components of. Distillation Experiment Class 9.
From antigua.desertcart.com
Buy ULTECHNOVO 1 Set Distillation Set Home Water Distiller Experiment Distillation Experiment Class 9 Steps for separation of ammonium chloride: There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. In this method, the mixture to be separated is heated and then cooled using a water condenser. Learn more in this ks3 chemistry guide from bitesize. Distillation is a separation technique used to remove a solvent from a mixture and keep. Distillation Experiment Class 9.
From www.oresomeresources.com
Fractional Distillation of Crude Oil experiment Oresome Resources Distillation Experiment Class 9 What is a simple distillation experiment? In this method, the mixture to be separated is heated and then cooled using a water condenser. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Take the mixture of sand,. Distillation Experiment Class 9.
From www.pinterest.co.uk
Distillation Process Science HowThingsWork Gif Distillation Distillation Experiment Class 9 In this method, the mixture to be separated is heated and then cooled using a water condenser. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Aim to separate the components of a mixture of sand, common salt. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Investigate the. Distillation Experiment Class 9.
From www.chegg.com
Hi, please help me with my lab report. My questions Distillation Experiment Class 9 Investigate the separation of water from a coloured solution using this simple distillation video, including. What is a simple distillation experiment? In this method, the mixture to be separated is heated and then cooled using a water condenser. Steps for separation of ammonium chloride: Distillation is a separation technique used to remove a solvent from a mixture and keep it.. Distillation Experiment Class 9.
From vectormine.com
Fractional distillation vector illustration VectorMine Distillation Experiment Class 9 Aim to separate the components of a mixture of sand, common salt. Steps for separation of ammonium chloride: In this method, the mixture to be separated is heated and then cooled using a water condenser. Investigate the separation of water from a coloured solution using this simple distillation video, including. Distillation refers to the selective boiling and subsequent condensation of. Distillation Experiment Class 9.
From phdessay.com
Fractional Distillation Experiment (300 Words) Distillation Experiment Class 9 What is a simple distillation experiment? Simple distillation separates components from their liquid mixtures based on their boiling point differences. Steps for separation of ammonium chloride: In this method, the mixture to be separated is heated and then cooled using a water condenser. Investigate the separation of water from a coloured solution using this simple distillation video, including. Learn more. Distillation Experiment Class 9.
From www.pinterest.com
Distillation Apparatus Diagram with full process and lab tools Distillation Experiment Class 9 Distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from bitesize. In this method, the mixture to be separated is heated and then cooled using a water condenser. What is a simple distillation experiment? Aim to separate the components of a mixture of sand, common salt.. Distillation Experiment Class 9.
From studylib.net
Experiment 300 Distillation FAMU Distillation Experiment Class 9 What is a simple distillation experiment? Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Investigate the separation of water from a coloured solution using this simple distillation video, including. Steps for separation of ammonium chloride: Learn more. Distillation Experiment Class 9.
From ar.inspiredpencil.com
Distillation Separating Mixtures Distillation Experiment Class 9 Take the mixture of sand, ammonium chloride and common salt in a china dish and. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Steps for separation of ammonium chloride: Simple distillation separates components from their liquid mixtures based on their boiling point differences. What is a simple distillation experiment? Investigate the separation. Distillation Experiment Class 9.
From edu.rsc.org
Simple distillation practical videos 1416 students Resource Distillation Experiment Class 9 Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. What is a simple distillation experiment? Investigate the separation of water from a coloured solution using this simple distillation video, including. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Take the mixture of sand, ammonium chloride and common salt. Distillation Experiment Class 9.
From www.shutterstock.com
533 Fractional Distillation Bilder, Stockfotos und Distillation Experiment Class 9 What is a simple distillation experiment? Steps for separation of ammonium chloride: Simple distillation separates components from their liquid mixtures based on their boiling point differences. In this method, the mixture to be separated is heated and then cooled using a water condenser. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Learn more in this. Distillation Experiment Class 9.
From oneclass.com
OneClass A student obtains the following data in the distillation Distillation Experiment Class 9 There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. In this method, the mixture to be separated is heated and then cooled using a water condenser. What is a simple distillation experiment? Investigate the separation of water from a coloured solution using this simple distillation video, including. Distillation refers to the selective boiling and subsequent condensation. Distillation Experiment Class 9.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Experiment Class 9 What is a simple distillation experiment? Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Learn more in this ks3 chemistry guide from bitesize. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. In. Distillation Experiment Class 9.
From www.differencebetween.com
Difference Between Fractional and Simple Distillation Compare the Distillation Experiment Class 9 Learn more in this ks3 chemistry guide from bitesize. Aim to separate the components of a mixture of sand, common salt. Take the mixture of sand, ammonium chloride and common salt in a china dish and. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. There are different ways to separate mixtures, for. Distillation Experiment Class 9.