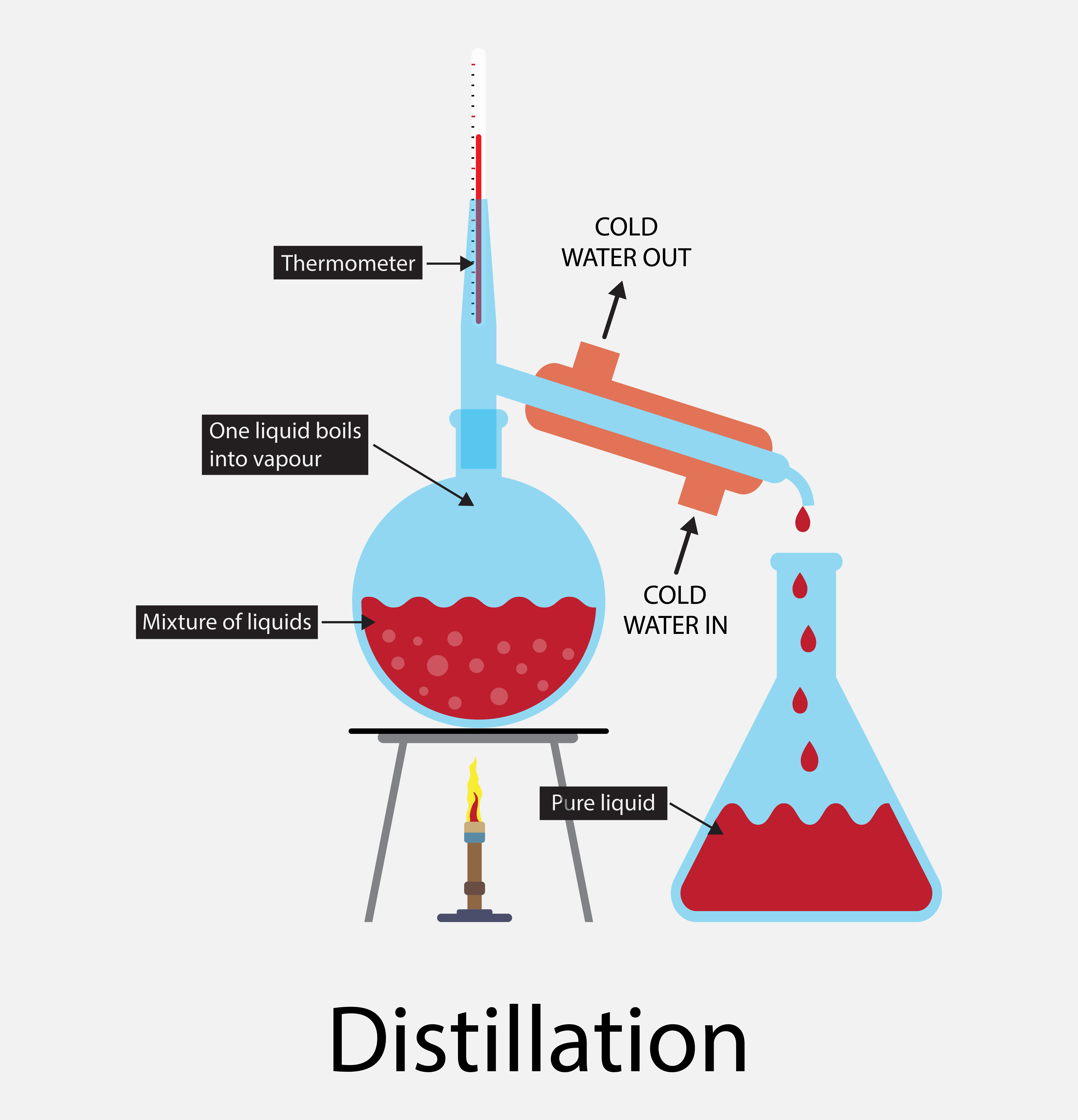
How To Distill Solvents . There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Learn more in this ks3 chemistry guide from. Instructions and descriptions for the. However, simple distillation requires very careful control of the. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. Record the temperature where liquid is. Apply the heat source to the distilling flask. Turn on the condenser water. Collect distillate at a rate of 1 drop per second. A mixture of two liquids can be separated by either distillation method. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Purification and recovery of organic solvents by distillation is a common laboratory operation.
from primaryleap.co.uk
There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. Instructions and descriptions for the. Record the temperature where liquid is. A mixture of two liquids can be separated by either distillation method. Learn more in this ks3 chemistry guide from. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Collect distillate at a rate of 1 drop per second. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Apply the heat source to the distilling flask.
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap
How To Distill Solvents A mixture of two liquids can be separated by either distillation method. Purification and recovery of organic solvents by distillation is a common laboratory operation. A mixture of two liquids can be separated by either distillation method. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Instructions and descriptions for the. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Record the temperature where liquid is. Apply the heat source to the distilling flask. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Learn more in this ks3 chemistry guide from. Turn on the condenser water. Collect distillate at a rate of 1 drop per second. However, simple distillation requires very careful control of the.
From www.pinterest.com
Making Essential Oils Distillation, ColdPress Extraction and Solvent How To Distill Solvents Record the temperature where liquid is. However, simple distillation requires very careful control of the. Collect distillate at a rate of 1 drop per second. Apply the heat source to the distilling flask. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. There are. How To Distill Solvents.
From future4200.com
How to distill solvents Solvent Recovery Future4200 How To Distill Solvents Learn more in this ks3 chemistry guide from. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Turn on the condenser water. Purification and recovery of organic solvents by distillation is a common laboratory operation. However, simple distillation requires very careful control of the. Record the temperature where liquid is. Distillation is a separation technique used. How To Distill Solvents.
From www.thoughtco.com
What Is Distillation? Principles and Uses How To Distill Solvents Instructions and descriptions for the. Purification and recovery of organic solvents by distillation is a common laboratory operation. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. However, simple distillation requires very careful control of the. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Turn on. How To Distill Solvents.
From www.mdpi.com
Water Free FullText LiquidLiquid Continuous Extraction and How To Distill Solvents Distillation is a separation technique used to remove a solvent from a mixture and keep it. Turn on the condenser water. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Instructions and descriptions for the. A mixture of two liquids can be separated by either distillation method. However, simple distillation requires very careful control of the.. How To Distill Solvents.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds How To Distill Solvents Collect distillate at a rate of 1 drop per second. However, simple distillation requires very careful control of the. A mixture of two liquids can be separated by either distillation method. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Turn on the condenser water. The preferred method for solvent removal in the. How To Distill Solvents.
From medium.com
‘Ultrapure Water and Distilled Water — What is the Difference? by How To Distill Solvents Distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is. Apply the heat source to the distilling. How To Distill Solvents.
From www.researchgate.net
Distillation of the anhydrous solvent from sodium... Download How To Distill Solvents However, simple distillation requires very careful control of the. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Apply the heat source to the distilling flask. Turn on the condenser water. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known. How To Distill Solvents.
From en.wikipedia.org
Distillation Wikipedia How To Distill Solvents Purification and recovery of organic solvents by distillation is a common laboratory operation. Learn more in this ks3 chemistry guide from. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Apply the heat source to the distilling flask. Instructions and descriptions for the. Collect distillate at a rate of. How To Distill Solvents.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii How To Distill Solvents Purification and recovery of organic solvents by distillation is a common laboratory operation. Apply the heat source to the distilling flask. Instructions and descriptions for the. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Record the temperature where liquid is. Learn more in this ks3 chemistry guide from. Turn on the condenser water. Distillation is. How To Distill Solvents.
From speichim.com
Main principes of distillation Speichim Processing Valls Química How To Distill Solvents Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. A mixture of two liquids can be separated by either distillation method. Apply the heat source to the distilling flask. Purification and recovery of organic solvents by distillation is a common laboratory operation. There are different ways to separate mixtures,. How To Distill Solvents.
From chem.libretexts.org
5.2C StepbyStep Procedures Chemistry LibreTexts How To Distill Solvents The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Record the temperature where liquid is. Apply the heat source to the distilling flask. Distillation is a separation technique used to remove. How To Distill Solvents.
From easywayscience78.blogspot.com
Distillation Easy way to learn science How To Distill Solvents However, simple distillation requires very careful control of the. Learn more in this ks3 chemistry guide from. Turn on the condenser water. Instructions and descriptions for the. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. Record the temperature where liquid is. A mixture. How To Distill Solvents.
From wikihow.com
How to Distill 12 Steps (with Pictures) wikiHow How To Distill Solvents Distillation is a separation technique used to remove a solvent from a mixture and keep it. Record the temperature where liquid is. Turn on the condenser water. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. Instructions and descriptions for the. Purification and recovery. How To Distill Solvents.
From www.deltat.com
Solvent Distillation Delta T How To Distill Solvents The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. A mixture of two liquids can be separated by either distillation method. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. However, simple distillation requires very careful control of the. Turn. How To Distill Solvents.
From www.wermac.org
Distillation Column Basic Distillation Equipment and Operation How To Distill Solvents Record the temperature where liquid is. Collect distillate at a rate of 1 drop per second. Purification and recovery of organic solvents by distillation is a common laboratory operation. A mixture of two liquids can be separated by either distillation method. However, simple distillation requires very careful control of the. Distillation is a separation technique used to remove a solvent. How To Distill Solvents.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry How To Distill Solvents A mixture of two liquids can be separated by either distillation method. Turn on the condenser water. Instructions and descriptions for the. Learn more in this ks3 chemistry guide from. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Apply the heat source to the distilling flask. Distillation is. How To Distill Solvents.
From primaryleap.co.uk
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap How To Distill Solvents Instructions and descriptions for the. Learn more in this ks3 chemistry guide from. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Apply the heat source to the distilling flask. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. Turn. How To Distill Solvents.
From extractiongradesolvents.com
Extractive Distillation Types of Distillation How To Distill Solvents Learn more in this ks3 chemistry guide from. Instructions and descriptions for the. Collect distillate at a rate of 1 drop per second. A mixture of two liquids can be separated by either distillation method. Purification and recovery of organic solvents by distillation is a common laboratory operation. Apply the heat source to the distilling flask. However, simple distillation requires. How To Distill Solvents.
From www.chemicals.co.uk
What Is Purified Water? The Chemistry Blog How To Distill Solvents Apply the heat source to the distilling flask. Purification and recovery of organic solvents by distillation is a common laboratory operation. A mixture of two liquids can be separated by either distillation method. Instructions and descriptions for the. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation is a technique for separating. How To Distill Solvents.
From chemistryguru.com.sg
Oxidation of Primary Alcohol to Aldehyde How To Distill Solvents Learn more in this ks3 chemistry guide from. Purification and recovery of organic solvents by distillation is a common laboratory operation. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Instructions and descriptions for the. Record. How To Distill Solvents.
From www.youtube.com
GCSE Chemistry 19 Simple and Fractional Distillation YouTube How To Distill Solvents Turn on the condenser water. A mixture of two liquids can be separated by either distillation method. However, simple distillation requires very careful control of the. Learn more in this ks3 chemistry guide from. Apply the heat source to the distilling flask. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Purification and recovery of organic. How To Distill Solvents.
From www.vrogue.co
Solvent Extraction Plant Distillation Section vrogue.co How To Distill Solvents However, simple distillation requires very careful control of the. Record the temperature where liquid is. Apply the heat source to the distilling flask. Purification and recovery of organic solvents by distillation is a common laboratory operation. Instructions and descriptions for the. Learn more in this ks3 chemistry guide from. A mixture of two liquids can be separated by either distillation. How To Distill Solvents.
From glossary.periodni.com
Download distillation.png image from How To Distill Solvents Purification and recovery of organic solvents by distillation is a common laboratory operation. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. Turn on the condenser water. However, simple distillation requires very careful control of. How To Distill Solvents.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts How To Distill Solvents Learn more in this ks3 chemistry guide from. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. A mixture of two liquids can be separated by either distillation method. Purification and recovery of organic solvents by distillation is a common laboratory operation. However, simple distillation requires very careful control. How To Distill Solvents.
From hubpages.com
How To Essential Oil Solvent Extraction HubPages How To Distill Solvents Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator is. Learn more in this ks3 chemistry guide from. Apply the heat source to the distilling. How To Distill Solvents.
From future4200.com
How to distill solvents Solvent Recovery Future4200 How To Distill Solvents There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Turn on the condenser water. Distillation is a separation technique used to remove a solvent from a mixture and keep it. A mixture of two liquids can be separated by either distillation method. Purification and recovery of organic solvents by distillation is a common laboratory operation. Record. How To Distill Solvents.
From www.youtube.com
Animated Water Distillation process diagram in PowerPoint / Chemistry How To Distill Solvents Collect distillate at a rate of 1 drop per second. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Apply the heat source to the distilling flask. Purification and recovery of organic solvents by distillation is a common laboratory operation. The preferred method for solvent removal in the laboratory is by use of. How To Distill Solvents.
From istsurface.com
Solvent Distillation System IST International Surface Technologies How To Distill Solvents There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Instructions and descriptions for the. Collect distillate at a rate of 1 drop per second. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from. Distillation is a technique for separating liquids from. How To Distill Solvents.
From www.pinterest.co.kr
Making Essential Oils Distillation, ColdPress Extraction and Solvent How To Distill Solvents Instructions and descriptions for the. Record the temperature where liquid is. A mixture of two liquids can be separated by either distillation method. Purification and recovery of organic solvents by distillation is a common laboratory operation. The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (also known as a rotovap), a rotary evaporator. How To Distill Solvents.
From www.youtube.com
DISTILLATION OF WATER AND ETHANOL YouTube How To Distill Solvents Purification and recovery of organic solvents by distillation is a common laboratory operation. However, simple distillation requires very careful control of the. Record the temperature where liquid is. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. There are different ways to separate mixtures, for example by filtration, crystallisation,. How To Distill Solvents.
From byjus.com
Refining Process Against Impurities Oil Refining Process Chemistry How To Distill Solvents Apply the heat source to the distilling flask. However, simple distillation requires very careful control of the. Purification and recovery of organic solvents by distillation is a common laboratory operation. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation is a technique for separating liquids from one another, using the differences in. How To Distill Solvents.
From future4200.com
How to distill solvents Page 5 Solvent Recovery Future4200 How To Distill Solvents Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Learn more in this ks3 chemistry guide from. Purification and recovery of organic solvents by distillation is a common laboratory operation. Record the temperature where liquid is. A mixture of two liquids can be separated by either distillation method. The. How To Distill Solvents.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts How To Distill Solvents Instructions and descriptions for the. Apply the heat source to the distilling flask. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. Learn more in this ks3 chemistry guide from. There are different. How To Distill Solvents.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog How To Distill Solvents Record the temperature where liquid is. Collect distillate at a rate of 1 drop per second. Instructions and descriptions for the. Distillation is a technique for separating liquids from one another, using the differences in boiling point between the individual components. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Learn more in this ks3 chemistry. How To Distill Solvents.
From future4200.com
How to distill solvents Solvent Recovery Future4200 How To Distill Solvents There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Record the temperature where liquid is. Purification and recovery of organic solvents by distillation is a common laboratory operation. Collect distillate at a rate of 1 drop per second. Instructions and descriptions for the. Turn on the condenser water. A mixture of two liquids can be separated. How To Distill Solvents.