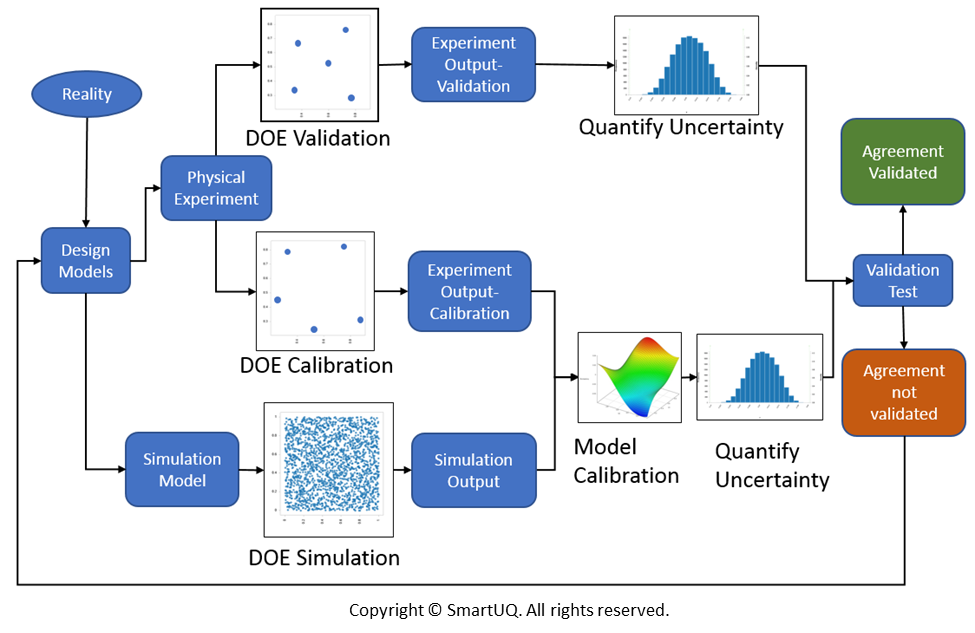
Medical Device V&V . A pivotal juncture in the development process is the design. We also provide a link to our. They are essential processes for ensuring the product's quality, reliability,. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. Toltec ventures helps medical device. Medical device verification and validation (v&v) are crucial steps in the medical device development process. In medical device development, v&v stands for design verification and design validation. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. But what’s the difference between the two, and why do we need them?
from www.smartuq.com
We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. But what’s the difference between the two, and why do we need them? Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. A pivotal juncture in the development process is the design. Medical device verification and validation (v&v) are crucial steps in the medical device development process. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. They are essential processes for ensuring the product's quality, reliability,. In medical device development, v&v stands for design verification and design validation. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. Toltec ventures helps medical device.
Medical Device Industries SmartUQ
Medical Device V&V But what’s the difference between the two, and why do we need them? They are essential processes for ensuring the product's quality, reliability,. Medical device verification and validation (v&v) are crucial steps in the medical device development process. But what’s the difference between the two, and why do we need them? A pivotal juncture in the development process is the design. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. We also provide a link to our. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. In medical device development, v&v stands for design verification and design validation. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. Toltec ventures helps medical device.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Medical Device V&V Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. A pivotal juncture in the development process is the design. But what’s the difference between the two, and why do we need them? In medical device development, v&v stands for design verification and design validation. We also provide a link to our. Toltec ventures helps. Medical Device V&V.
From medium.com
The 3 FDA medical device classes [differences and examples explained Medical Device V&V Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. We also provide a link to our. A pivotal juncture in the development process is the design. Toltec ventures helps. Medical Device V&V.
From www.vrogue.co
The Medical Device Development Process The Red Outlin vrogue.co Medical Device V&V They are essential processes for ensuring the product's quality, reliability,. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. But what’s the difference between the two, and why do we need them? Toltec ventures helps medical device. Medical device verification and validation (v&v) are crucial steps in. Medical Device V&V.
From rs-ness.com
Process Validation Pharma vs. Medical Device RS NESS Medical Device V&V We also provide a link to our. But what’s the difference between the two, and why do we need them? Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss. Medical Device V&V.
From www.oxfordcc.co.uk
Software as a Medical Device Oxford Computer Consultants Medical Device V&V Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. We also provide a link to our. Toltec ventures helps medical device. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. They are essential processes for ensuring the product's. Medical Device V&V.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device V&V We also provide a link to our. They are essential processes for ensuring the product's quality, reliability,. A pivotal juncture in the development process is the design. But what’s the difference between the two, and why do we need them? In medical device development, v&v stands for design verification and design validation. Assuring that the computational model has been formed. Medical Device V&V.
From enterprisepeak.com
Medical Devices from Concept to Commercialization Enterprise Peak Medical Device V&V In medical device development, v&v stands for design verification and design validation. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. Medical device verification and validation (v&v) are crucial steps in the medical device development process. Bringing a medical device to market demands an unerring dedication to safety, quality,. Medical Device V&V.
From www.slideteam.net
Medical Device Process Validation Flowchart Medical Device V&V This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. Assuring that the computational model has been formed using sound procedures is key and is achieved through the. Medical Device V&V.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device V&V We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. They are essential processes for ensuring the product's quality, reliability,. Medical device verification and validation (v&v) are crucial steps in the medical device. Medical Device V&V.
From www.youtube.com
MAVIS Medical Device V&V Automation Framework YouTube Medical Device V&V We also provide a link to our. In medical device development, v&v stands for design verification and design validation. But what’s the difference between the two, and why do we need them? Medical device verification and validation (v&v) are crucial steps in the medical device development process. Toltec ventures helps medical device. This article defines software verification and validation for. Medical Device V&V.
From www.youtube.com
MAVIS Medical Device V&V Automation Framework Waveform Verification Medical Device V&V We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. Medical device verification and validation (v&v) are crucial steps in the medical device development process. We also provide a link to our. This article defines software verification and validation for medical devices and provides an overview of ce. Medical Device V&V.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device V&V We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. They are essential processes for ensuring the product's quality, reliability,. Medical device verification and validation (v&v) are crucial steps in the medical device development process. Assuring that the computational model has been formed using sound procedures is key. Medical Device V&V.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device V&V Medical device verification and validation (v&v) are crucial steps in the medical device development process. We also provide a link to our. But what’s the difference between the two, and why do we need them? We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. A pivotal juncture. Medical Device V&V.
From pharmatreasures.blogspot.com
Pharma Treasures V Model Validation Concept in Pharmaceuticals Medical Device V&V Toltec ventures helps medical device. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. In medical device development, v&v stands for design verification and design validation. We. Medical Device V&V.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device V&V Toltec ventures helps medical device. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. But what’s the difference between the two, and why do we need them? We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest. Medical Device V&V.
From www.smartuq.com
Medical Device Industries SmartUQ Medical Device V&V Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. A pivotal juncture in the development process is the design. We also provide a link to our. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. But what’s the difference between the. Medical Device V&V.
From www.joharidigital.com
Medical Device Design and Development The Ultimate Guide from scratch Medical Device V&V We also provide a link to our. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. Medical device verification and validation (v&v) are crucial steps in the medical device development process. Toltec ventures helps medical device. They are essential processes for ensuring the product's. Medical Device V&V.
From perfectusbiomed.com
Supporting you on your medical device product development journey Medical Device V&V They are essential processes for ensuring the product's quality, reliability,. Medical device verification and validation (v&v) are crucial steps in the medical device development process. But what’s the difference between the two, and why do we need them? Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation. Medical Device V&V.
From bluefruit.co.uk
V&V Testing Signs you have a verification & validation issue Medical Device V&V Toltec ventures helps medical device. In medical device development, v&v stands for design verification and design validation. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. Medical device verification and validation (v&v) are crucial steps in the medical device development process. But what’s the difference between the two, and. Medical Device V&V.
From www.semiconductorforu.com
LMD announces VSensor™ wearables, smartphones and mobile devices with Medical Device V&V In medical device development, v&v stands for design verification and design validation. A pivotal juncture in the development process is the design. We also provide a link to our. Toltec ventures helps medical device. Medical device verification and validation (v&v) are crucial steps in the medical device development process. Assuring that the computational model has been formed using sound procedures. Medical Device V&V.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device V&V Toltec ventures helps medical device. But what’s the difference between the two, and why do we need them? In medical device development, v&v stands for design verification and design validation. We also provide a link to our. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. Medical device verification. Medical Device V&V.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device V&V Medical device verification and validation (v&v) are crucial steps in the medical device development process. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. Toltec ventures helps medical device. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation. Medical Device V&V.
From mavink.com
Examples Of Medical Devices Medical Device V&V But what’s the difference between the two, and why do we need them? In medical device development, v&v stands for design verification and design validation. A pivotal juncture in the development process is the design. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. This article defines software verification and validation for medical devices. Medical Device V&V.
From www.sqs.es
Harmonized Standards Medical Device Software MDR/IVDR SQS Medical Device V&V Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. A pivotal juncture in the development process is the design. Toltec ventures helps medical device. We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. This article defines software verification and validation for. Medical Device V&V.
From vem-medical.com
Medical Device Manufacturing Medical Device V&V Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. But what’s the difference between the two, and why do we need them? Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. They are essential processes for ensuring the. Medical Device V&V.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Medical Device V&V Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. Toltec ventures helps medical device. In medical device development, v&v stands for design verification and design validation. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. We also provide. Medical Device V&V.
From www.slideteam.net
Stages Of Medical Device And Drug Development PPT PowerPoint Medical Device V&V This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification.. Medical Device V&V.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device V&V We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. Toltec ventures helps medical device. A pivotal juncture in the development process is the design. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and. Medical Device V&V.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device V&V They are essential processes for ensuring the product's quality, reliability,. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy. A pivotal juncture in the development process is the design.. Medical Device V&V.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog Medical Device V&V This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. Bringing a medical device to market demands an unerring dedication to safety, quality, and efficacy.. Medical Device V&V.
From medicaldialogues.in
Medical devices of all four categories will be brought under regulation Medical Device V&V Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. They are essential processes for ensuring the product's quality, reliability,. Bringing a medical device to. Medical Device V&V.
From clin-r.com
EU MDR how to structure your Medical Device Technical Document Clin R Medical Device V&V Toltec ventures helps medical device. This article defines software verification and validation for medical devices and provides an overview of ce marking and 510k requirements. We also provide a link to our. But what’s the difference between the two, and why do we need them? Assuring that the computational model has been formed using sound procedures is key and is. Medical Device V&V.
From japaneseclass.jp
Images of ソリトン JapaneseClass.jp Medical Device V&V We also provide a link to our. But what’s the difference between the two, and why do we need them? We sat down with v&v expert byron larson, president of toltec ventures llc, to discuss the latest trends in validation and verification. A pivotal juncture in the development process is the design. This article defines software verification and validation for. Medical Device V&V.
From www.greenlight.guru
Medical Device Development [Understanding the 5 Phases] Medical Device V&V Medical device verification and validation (v&v) are crucial steps in the medical device development process. In medical device development, v&v stands for design verification and design validation. A pivotal juncture in the development process is the design. But what’s the difference between the two, and why do we need them? Toltec ventures helps medical device. We also provide a link. Medical Device V&V.
From www.openaccessgovernment.org
Medical devices Technology addressing medical wellbeing Medical Device V&V They are essential processes for ensuring the product's quality, reliability,. Assuring that the computational model has been formed using sound procedures is key and is achieved through the processes of verification, validation and uncertainty quantification. A pivotal juncture in the development process is the design. Toltec ventures helps medical device. But what’s the difference between the two, and why do. Medical Device V&V.