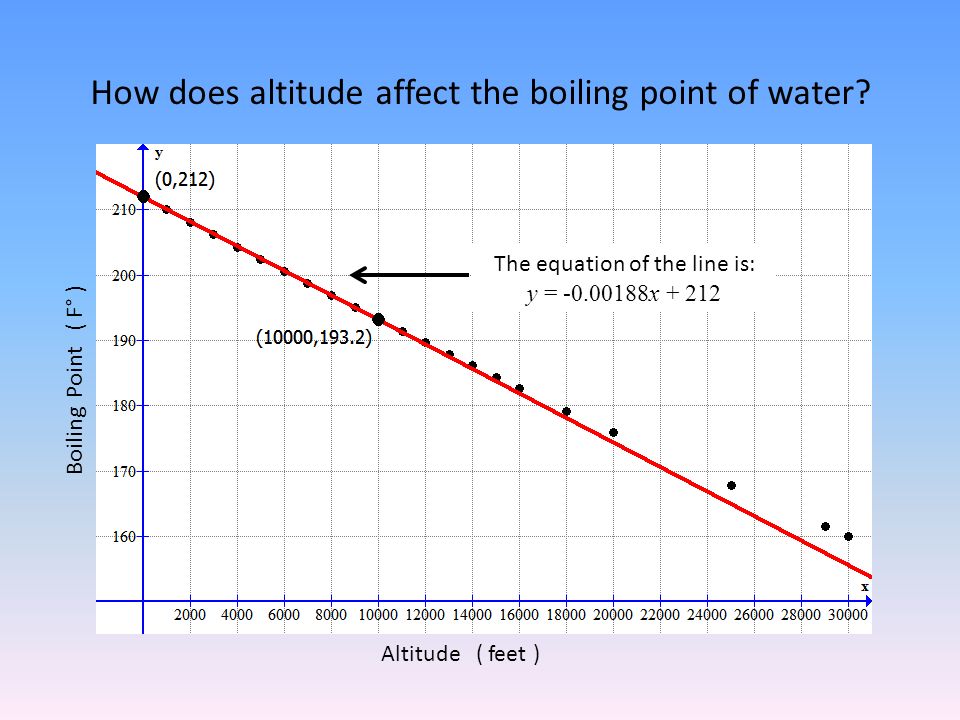
Water Boiling Point High Altitude . Generally, water will boil faster at higher altitudes due to the lower atmospheric pressure. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the At what temperature does water. The height of mount everest in nepal is 8848 m. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. According the table above the boiling point of water is aprox. This means that foods may require. It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? This article explores how the boiling point of water changes with increasing. The boiling point of water is affected by altitude above sea level. Find below the calculators to. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. 127 rows the boiling point of water decreases with altitude:
from bceweb.org
This means that foods may require. At what temperature does water. According the table above the boiling point of water is aprox. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). 127 rows the boiling point of water decreases with altitude: It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? Find below the calculators to. The boiling point of water is affected by altitude above sea level. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the
Water Boiling Point Vs Pressure Chart A Visual Reference of Charts
Water Boiling Point High Altitude At what temperature does water. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. According the table above the boiling point of water is aprox. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. This means that foods may require. The boiling point of water is affected by altitude above sea level. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? At what temperature does water. 127 rows the boiling point of water decreases with altitude: The height of mount everest in nepal is 8848 m. Generally, water will boil faster at higher altitudes due to the lower atmospheric pressure. Find below the calculators to. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. This article explores how the boiling point of water changes with increasing. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the
From socratic.org
How do mountains affect temperature on both the windward side and the Water Boiling Point High Altitude The boiling point of water is affected by altitude above sea level. At what temperature does water. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. Boiling point is also defined as a. Water Boiling Point High Altitude.
From blog.thermoworks.com
High Altitude and Its Effects on Cooking ThermoWorks Water Boiling Point High Altitude At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). Find below the calculators to. According the table above the boiling point of water is aprox. The height of mount everest in nepal is 8848 m. This article explores how the boiling point of water changes with increasing. As the altitude increases, the atmospheric pressure pushing. Water Boiling Point High Altitude.
From www.youtube.com
Boiling point of oil is greater than water ? YouTube Water Boiling Point High Altitude Find below the calculators to. Generally, water will boil faster at higher altitudes due to the lower atmospheric pressure. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). 127 rows the boiling point of water decreases with altitude: As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the This means. Water Boiling Point High Altitude.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Point Tessshebaylo Water Boiling Point High Altitude Generally, water will boil faster at higher altitudes due to the lower atmospheric pressure. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. This article explores how the boiling point of water changes with increasing. At what temperature does water. This means that foods may require. The boiling point. Water Boiling Point High Altitude.
From www.pinterest.com
Boiling Point of Water and Altitude Boiling Point, Food Guide, Cookery Water Boiling Point High Altitude The boiling point of water is affected by altitude above sea level. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. This means that foods may require. This article explores how the boiling point. Water Boiling Point High Altitude.
From mavink.com
Water Boiling Point Pressure Chart Water Boiling Point High Altitude Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. According the table above the boiling point of water is aprox. It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? The boiling point of water is affected by altitude above. Water Boiling Point High Altitude.
From in.pinterest.com
How does altitude affect boiling? Vapor pressure and Atmospheric Water Boiling Point High Altitude Find below the calculators to. According the table above the boiling point of water is aprox. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the 127 rows the boiling point of water decreases with altitude: At what temperature does water. This means that foods may require. One of the most significant changes that occur. Water Boiling Point High Altitude.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Water Boiling Point High Altitude The boiling point of water is affected by altitude above sea level. This means that foods may require. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the This article explores how the boiling point of water changes with increasing. Find below the calculators to. At sea level, higher atmospheric pressure means that liquid h2o. Water Boiling Point High Altitude.
From www.youtube.com
Study Boiling Point of Water with Altitude YouTube Water Boiling Point High Altitude Find below the calculators to. The height of mount everest in nepal is 8848 m. The boiling point of water is affected by altitude above sea level. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. One of the most significant changes that occur in high altitude areas concerning. Water Boiling Point High Altitude.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Water Boiling Point High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. Find below the calculators to. At what temperature does water. It is commonly known that water boils at a. Water Boiling Point High Altitude.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Tessshebaylo Water Boiling Point High Altitude According the table above the boiling point of water is aprox. Generally, water will boil faster at higher altitudes due to the lower atmospheric pressure. This article explores how the boiling point of water changes with increasing. The height of mount everest in nepal is 8848 m. One of the most significant changes that occur in high altitude areas concerning. Water Boiling Point High Altitude.
From www.asianbeernetwork.com
Boiling at Altitude Brewing Up High Asian Beer Network Water Boiling Point High Altitude At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. The height of mount everest in nepal is 8848 m. Find below the calculators to. It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? The boiling point of water. Water Boiling Point High Altitude.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Water Boiling Point High Altitude This means that foods may require. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water.. Water Boiling Point High Altitude.
From www.alamy.com
Atmospheric pressure alters the boiling point of water Stock Photo Alamy Water Boiling Point High Altitude The boiling point of water is affected by altitude above sea level. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. According the table above the boiling point of. Water Boiling Point High Altitude.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Water Boiling Point High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? Boiling point is also defined as. Water Boiling Point High Altitude.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Water Boiling Point High Altitude As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the The height of mount everest in nepal is 8848 m. Find below the calculators to. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. At an altitude of 5,000 feet (1,524 meters), water boils. Water Boiling Point High Altitude.
From www.numerade.com
SOLVEDIn the century, explorers used the boiling point Water Boiling Point High Altitude This article explores how the boiling point of water changes with increasing. Find below the calculators to. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. 127 rows the boiling point of water. Water Boiling Point High Altitude.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Water Boiling Point High Altitude At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). Find below the calculators to. This article explores how the boiling point of water changes with increasing. At what temperature does water. The height of mount everest in nepal is 8848 m. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows. Water Boiling Point High Altitude.
From www.instructables.com
Boiling Water Bath Canning 12 Steps Instructables Water Boiling Point High Altitude Generally, water will boil faster at higher altitudes due to the lower atmospheric pressure. This article explores how the boiling point of water changes with increasing. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the 127 rows the boiling point of water decreases with altitude: It is commonly known that water boils at a. Water Boiling Point High Altitude.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level Water Boiling Point High Altitude The boiling point of water is affected by altitude above sea level. Find below the calculators to. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. This article explores how the boiling point of water changes with increasing. 127 rows the boiling point of water decreases with altitude:. Water Boiling Point High Altitude.
From aweseas.blogspot.com
Boiling Point Of Water Sea Level Water Boiling Point High Altitude As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the According the table above the boiling point of water is aprox. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. The boiling point of water is affected by altitude above sea level. The height. Water Boiling Point High Altitude.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs Water Boiling Point High Altitude The height of mount everest in nepal is 8848 m. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the According the table above the boiling point of water is aprox. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). This means that foods may require. One of the most. Water Boiling Point High Altitude.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Water Boiling Point High Altitude This article explores how the boiling point of water changes with increasing. It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. The boiling point of water is affected by. Water Boiling Point High Altitude.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Water Boiling Point High Altitude Find below the calculators to. The height of mount everest in nepal is 8848 m. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. As the altitude increases, the atmospheric pressure pushing down on. Water Boiling Point High Altitude.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Water Boiling Point High Altitude According the table above the boiling point of water is aprox. At what temperature does water. This article explores how the boiling point of water changes with increasing. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. 127 rows the boiling point of water decreases with altitude: Boiling point. Water Boiling Point High Altitude.
From www.youtube.com
22 Boiling Point Elevation Explanation YouTube Water Boiling Point High Altitude As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the 127 rows the boiling point of water decreases with altitude: At what temperature does water. This means that foods may require. According the table above the boiling point of water is aprox. Find below the calculators to. One of the most significant changes that occur. Water Boiling Point High Altitude.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes Water Boiling Point High Altitude 127 rows the boiling point of water decreases with altitude: As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the The boiling point of water is affected by altitude above sea level. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. It is. Water Boiling Point High Altitude.
From www.dreamstime.com
Boiling Point of Water in Different Altitude Meter Levels Outline Water Boiling Point High Altitude This means that foods may require. At what temperature does water. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). This article explores how the boiling point of water changes with increasing. Find below. Water Boiling Point High Altitude.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Water Boiling Point High Altitude According the table above the boiling point of water is aprox. The boiling point of water is affected by altitude above sea level. 127 rows the boiling point of water decreases with altitude: As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the Boiling point is also defined as a substance's highest possible temperature in. Water Boiling Point High Altitude.
From www.showme.com
ShowMe boiling point elevation Water Boiling Point High Altitude At what temperature does water. The height of mount everest in nepal is 8848 m. It is commonly known that water boils at a lower temperature at higher altitudes, but what is that temperature? This article explores how the boiling point of water changes with increasing. The boiling point of water is affected by altitude above sea level. According the. Water Boiling Point High Altitude.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry Water Boiling Point High Altitude The height of mount everest in nepal is 8848 m. At what temperature does water. Generally, water will boil faster at higher altitudes due to the lower atmospheric pressure. At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the One of. Water Boiling Point High Altitude.
From facts.net
20 Fascinating Facts About Boiling Point Water Boiling Point High Altitude Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. This means that foods may require. According the table above the boiling point of water is aprox. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the 127 rows the boiling point of water decreases. Water Boiling Point High Altitude.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level Water Boiling Point High Altitude 127 rows the boiling point of water decreases with altitude: At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the The height of mount everest in nepal is 8848 m. It is commonly known. Water Boiling Point High Altitude.
From bceweb.org
Water Boiling Point Vs Pressure Chart A Visual Reference of Charts Water Boiling Point High Altitude At an altitude of 5,000 feet (1,524 meters), water boils at approximately 94.9°c (202.8°f). 127 rows the boiling point of water decreases with altitude: According the table above the boiling point of water is aprox. At sea level, higher atmospheric pressure means that liquid h2o turns into water vapor (and reaches boiling point) at a high. The height of mount. Water Boiling Point High Altitude.
From www.tastingtable.com
How To Adjust Your Water Boiling Process For High Altitudes Water Boiling Point High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. The boiling point of water is affected by altitude above sea level. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the The height of mount everest in nepal is 8848 m. According the table. Water Boiling Point High Altitude.