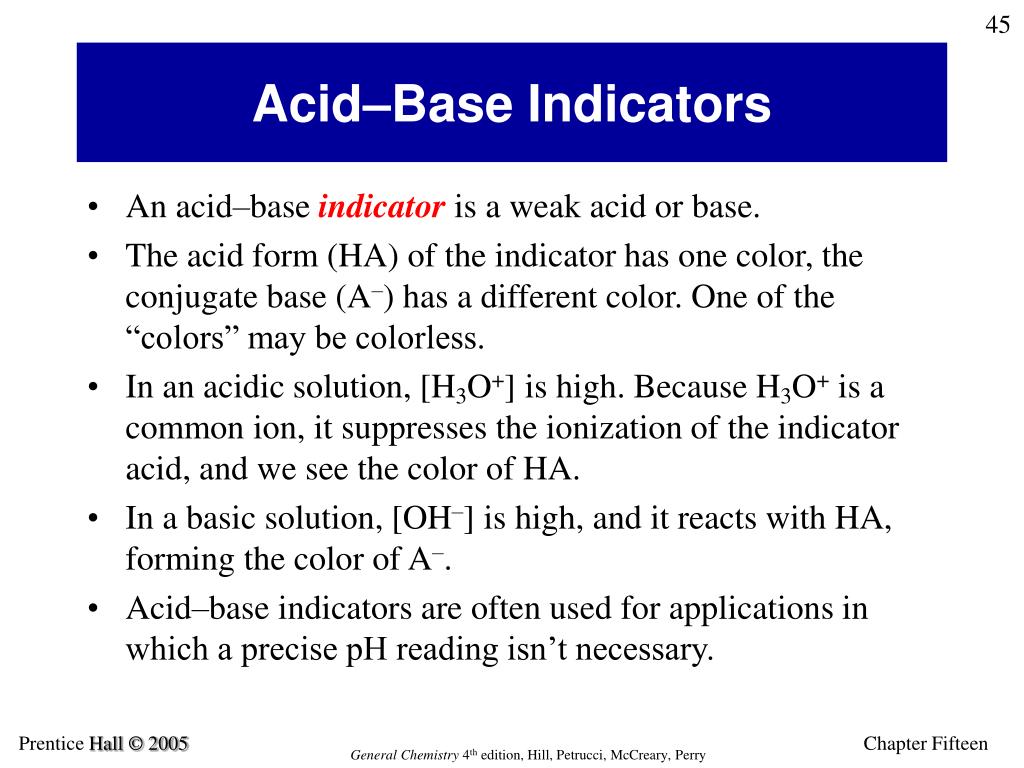
What Are Acid And Base Indicators Important . Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. This information is useful in a variety of fields, including chemistry, biology, and Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Determine the acidic dissociation constants ka or kai of indicators. An indicator does not change color from pure acid to pure alkaline at specific The undissociated form of the indicator is a different color than the iogenic form of the indicator. Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Indicators are substances whose solutions change color due to changes in ph.
from www.slideserve.com
Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Determine the acidic dissociation constants ka or kai of indicators. An indicator does not change color from pure acid to pure alkaline at specific Indicators are substances whose solutions change color due to changes in ph. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. This information is useful in a variety of fields, including chemistry, biology, and
PPT Acids, Bases, and AcidBase Equilibria PowerPoint Presentation
What Are Acid And Base Indicators Important Indicators are substances whose solutions change color due to changes in ph. Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. Because acidity and alkalinity relate to ph, they may also be known as ph indicators. This information is useful in a variety of fields, including chemistry, biology, and An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. Determine the acidic dissociation constants ka or kai of indicators. Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. An indicator does not change color from pure acid to pure alkaline at specific The undissociated form of the indicator is a different color than the iogenic form of the indicator. Indicators are substances whose solutions change color due to changes in ph.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Are Acid And Base Indicators Important An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. An indicator does not change color from pure acid to pure alkaline at specific Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 What Are Acid And Base Indicators Important Indicators are substances whose solutions change color due to changes in ph. This information is useful in a variety of fields, including chemistry, biology, and Determine the acidic dissociation constants ka or kai of indicators. Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Chemical indicator, any substance that gives a visible sign, usually. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT AcidBase Indicators PowerPoint Presentation, free download ID What Are Acid And Base Indicators Important Indicators are substances whose solutions change color due to changes in ph. Determine the acidic dissociation constants ka or kai of indicators. An indicator does not change color from pure acid to pure alkaline at specific Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of. What Are Acid And Base Indicators Important.
From www.teachoo.com
Uses of Acids 10+ Examples (with Images) Teachoo Teachoo Questio What Are Acid And Base Indicators Important Determine the acidic dissociation constants ka or kai of indicators. Indicators are substances whose solutions change color due to changes in ph. The undissociated form of the indicator is a different color than the iogenic form of the indicator. This information is useful in a variety of fields, including chemistry, biology, and Chemical indicator, any substance that gives a visible. What Are Acid And Base Indicators Important.
From www.pinterest.com
Most of us, chemists or otherwise, have probably come across pH What Are Acid And Base Indicators Important Indicators are substances whose solutions change color due to changes in ph. The undissociated form of the indicator is a different color than the iogenic form of the indicator. An indicator does not change color from pure acid to pure alkaline at specific This information is useful in a variety of fields, including chemistry, biology, and An example is the. What Are Acid And Base Indicators Important.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ What Are Acid And Base Indicators Important Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. Indicators are substances whose. What Are Acid And Base Indicators Important.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ What Are Acid And Base Indicators Important The undissociated form of the indicator is a different color than the iogenic form of the indicator. Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Indicators are substances whose solutions change. What Are Acid And Base Indicators Important.
From www.oist.jp
pH Values of Common Substances Okinawa Institute of Science and What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Indicators are substances whose solutions change color due to changes in ph. This information is useful in a variety of fields, including chemistry, biology,. What Are Acid And Base Indicators Important.
From brainly.in
What are universal indicators? what are the effect of indicators on What Are Acid And Base Indicators Important Determine the acidic dissociation constants ka or kai of indicators. This information is useful in a variety of fields, including chemistry, biology, and Because acidity and alkalinity relate to ph, they may also be known as ph indicators. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Hin (aq) ⏟ red +. What Are Acid And Base Indicators Important.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry What Are Acid And Base Indicators Important Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Determine the acidic dissociation constants ka or kai of indicators. Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in. What Are Acid And Base Indicators Important.
From www.chemistry4students.com
Chemistry 4 Students Common AcidBase Indicators What Are Acid And Base Indicators Important Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. The undissociated form of the indicator is a different color than the iogenic form of the indicator. An indicator does not change color. What Are Acid And Base Indicators Important.
From brainly.in
How many types of acid base indicators are there? What are they What Are Acid And Base Indicators Important Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. This information is useful in a variety of fields, including chemistry, biology, and The undissociated form of the indicator is a different color. What Are Acid And Base Indicators Important.
From examples.yourdictionary.com
Difference Between Acids and Bases Key Properties What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. Determine the acidic dissociation constants ka or kai of indicators. An indicator does not change color from pure acid to pure alkaline at specific Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence. What Are Acid And Base Indicators Important.
From www.youtube.com
R3.1.14 / R3.1.15 Acidbase indicators (HL) YouTube What Are Acid And Base Indicators Important This information is useful in a variety of fields, including chemistry, biology, and Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. This information is useful in a variety of fields, including chemistry, biology, and Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Indicators are substances whose solutions change color due to changes in ph. Chemical. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID What Are Acid And Base Indicators Important Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. This information is useful in a variety of fields, including chemistry, biology, and An example is the substance called methyl yellow, which imparts. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. An indicator does not change color from pure acid to pure alkaline at specific The undissociated form of the indicator is a different color than the iogenic form of the indicator. Chemical indicator, any substance that gives a visible sign, usually. What Are Acid And Base Indicators Important.
From www.scribd.com
ACIDBASE INDICATORS 211.ppt Acid Ph What Are Acid And Base Indicators Important Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Determine the acidic dissociation constants ka or kai of indicators. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Hin. What Are Acid And Base Indicators Important.
From www.worksheetsplanet.com
Acid and Bases Differences What Are Acid And Base Indicators Important Because acidity and alkalinity relate to ph, they may also be known as ph indicators. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. An indicator does not change color from pure acid to pure alkaline at specific Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) +. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT Conjugate acids and bases PowerPoint Presentation, free download What Are Acid And Base Indicators Important An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. This information is useful in a variety of fields, including chemistry, biology, and The undissociated form of the indicator is a different color than the iogenic form of the indicator. Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq). What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT ACID BASE REACTIONS PowerPoint Presentation, free download ID What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Determine the acidic dissociation. What Are Acid And Base Indicators Important.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Are Acid And Base Indicators Important Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Because acidity and alkalinity relate to. What Are Acid And Base Indicators Important.
From www.teachoo.com
What are Olfactory Indicators? Give Examples Teachoo Teachoo Quest What Are Acid And Base Indicators Important This information is useful in a variety of fields, including chemistry, biology, and Indicators are substances whose solutions change color due to changes in ph. Determine the acidic dissociation constants ka or kai of indicators. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Chemical indicator, any substance that gives a visible. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT Conjugate acids and bases PowerPoint Presentation ID2837947 What Are Acid And Base Indicators Important Because acidity and alkalinity relate to ph, they may also be known as ph indicators. The undissociated form of the indicator is a different color than the iogenic form of the indicator. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o. What Are Acid And Base Indicators Important.
From www.chemedx.org
Making Natural Acid Base Indicators Chemical Education Xchange What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. This information is useful in a variety of fields, including chemistry, biology, and Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Indicators are substances whose solutions change color due to changes in ph. Chemical. What Are Acid And Base Indicators Important.
From brainly.in
what is an acidbase indicator? give one example. Brainly.in What Are Acid And Base Indicators Important Determine the acidic dissociation constants ka or kai of indicators. Because acidity and alkalinity relate to ph, they may also be known as ph indicators. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or. What Are Acid And Base Indicators Important.
From dokumen.tips
(PPT) Acid Base Indicators Experiment 6. What are acids and bases What Are Acid And Base Indicators Important Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Determine the acidic dissociation constants ka or kai of indicators. Indicators are substances whose solutions change color due to changes in ph. An. What Are Acid And Base Indicators Important.
From www.youtube.com
Science Class 7Acidbase indicators CBSE YouTube What Are Acid And Base Indicators Important The undissociated form of the indicator is a different color than the iogenic form of the indicator. This information is useful in a variety of fields, including chemistry, biology, and Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Indicators are substances whose solutions change color due to changes in ph. An indicator does. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT Acids, Bases, and AcidBase Equilibria PowerPoint Presentation What Are Acid And Base Indicators Important An indicator does not change color from pure acid to pure alkaline at specific This information is useful in a variety of fields, including chemistry, biology, and Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an. What Are Acid And Base Indicators Important.
From www.researchgate.net
Characteristics of the acidbase indicators Download Scientific Diagram What Are Acid And Base Indicators Important Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Because acidity and alkalinity relate to ph, they may also be known as ph indicators. An example is the substance called methyl yellow,. What Are Acid And Base Indicators Important.
From ar.inspiredpencil.com
Acids And Bases List Strength What Are Acid And Base Indicators Important This information is useful in a variety of fields, including chemistry, biology, and Indicators are substances whose solutions change color due to changes in ph. Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. An indicator does not change color from pure acid to pure alkaline at specific Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Indicators are substances whose solutions change color due to changes in ph.. What Are Acid And Base Indicators Important.
From mchsscience.weebly.com
Acids and Bases MCHS Science What Are Acid And Base Indicators Important An indicator does not change color from pure acid to pure alkaline at specific Because acidity and alkalinity relate to ph, they may also be known as ph indicators. An example is the substance called methyl yellow, which imparts a yellow colour to an alkaline solution. Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) +. What Are Acid And Base Indicators Important.
From www.howtopronounce.com
Take the free online AcidBase Indicators Color Change! Chemistry What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. Because acidity and alkalinity relate to ph, they may also be known as ph indicators. Indicators are substances whose solutions change color due to changes in ph. Chemical indicator, any substance that gives a visible sign, usually by a colour change,. What Are Acid And Base Indicators Important.
From www.slideserve.com
PPT ACIDBASE INDICATORS PowerPoint Presentation, free download ID What Are Acid And Base Indicators Important Hin (aq) ⏟ red + h 2o (l) ⇌ h 3o + (aq) + in − (aq) ⏟ yellow. Indicators are substances whose solutions change color due to changes in ph. This information is useful in a variety of fields, including chemistry, biology, and Chemical indicator, any substance that gives a visible sign, usually by a colour change, of the. What Are Acid And Base Indicators Important.