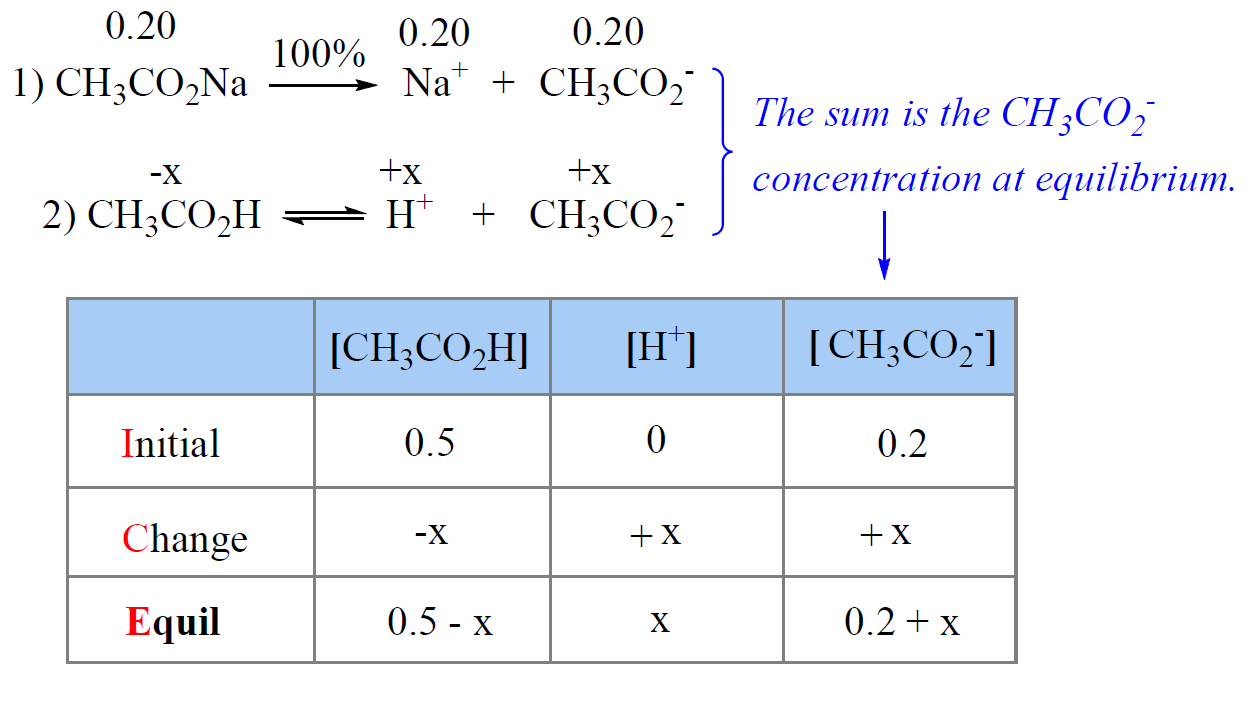
Adding Acid To Buffer Calculation . The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. Buffers allow chemists to maintain a specific ph range for a reaction. When a strong acid (h 3 o +) is added to a buffer solution the. Calculation of the ph of a buffer solution after addition of a small amount of acid. Calculate the ph of a buffer before and after the addition of added acid or. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution.
from general.chemistrysteps.com
Adding an acid to this buffer solution. Calculate the ph of a buffer before and after the addition of added acid or. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculation of the ph of a buffer solution after addition of a small amount of acid. Buffers allow chemists to maintain a specific ph range for a reaction. When a strong acid (h 3 o +) is added to a buffer solution the. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Hydrogen ions combine with the ethanoate ions to.
pH of a Buffer Solution Chemistry Steps
Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Calculation of the ph of a buffer solution after addition of a small amount of acid. Buffers allow chemists to maintain a specific ph range for a reaction. Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. When a strong acid (h 3 o +) is added to a buffer solution the. Calculate the ph of a buffer before and after the addition of added acid or. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution.
From www.youtube.com
pH calculation of a buffer solution made from a weak base and its Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Buffers allow chemists to maintain a specific ph range for a reaction. Adding an acid to this buffer solution. Calculation of the ph of a buffer solution after addition of a small amount of acid. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind. Adding Acid To Buffer Calculation.
From www.youtube.com
pH of a buffer after strong acid addition YouTube Adding Acid To Buffer Calculation The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Buffers allow chemists to maintain a specific ph range for a reaction. Calculate the ph of a buffer before and after the addition of added acid or. When a strong acid (h 3 o +) is added to a buffer solution the. Adding. Adding Acid To Buffer Calculation.
From classnotes.org.in
Calculation of pH of a Buffer Mixture Chemistry, Class 11, Ionic Adding Acid To Buffer Calculation Adding an acid to this buffer solution. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. The buffer solution must remove most of the new. Adding Acid To Buffer Calculation.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Adding Acid To Buffer Calculation Adding an acid to this buffer solution. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. When a strong acid (h 3 o +) is added to a buffer solution the. Hydrogen ions combine with the ethanoate ions to. Calculate the ph of a buffer before and after the addition of added. Adding Acid To Buffer Calculation.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube Adding Acid To Buffer Calculation Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. Buffers allow chemists to maintain a specific ph range for a reaction. Calculation of the ph of a buffer solution after addition of a small amount of acid. Calculate. Adding Acid To Buffer Calculation.
From www.youtube.com
Calculating pH changes to a buffer solution YouTube Adding Acid To Buffer Calculation Adding an acid to this buffer solution. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculate the ph of a buffer before and after the addition of. Adding Acid To Buffer Calculation.
From www.youtube.com
Adding Acid to a Buffer Calculating the pH using Henderson Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Calculation of the ph of a buffer solution after addition of a small amount of acid. When a strong acid (h 3 o +) is added to a buffer solution the. Adding. Adding Acid To Buffer Calculation.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Adding Acid To Buffer Calculation Adding an acid to this buffer solution. Calculate the ph of a buffer before and after the addition of added acid or. Calculation of the ph of a buffer solution after addition of a small amount of acid. Buffers allow chemists to maintain a specific ph range for a reaction. The buffer solution must remove most of the new hydrogen. Adding Acid To Buffer Calculation.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. The. Adding Acid To Buffer Calculation.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Adding Acid To Buffer Calculation Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. When a strong acid (h 3 o +) is added to a buffer solution the. The. Adding Acid To Buffer Calculation.
From dandkmotorsports.com
Tris Buffer Recipe Calculator Dandk Organizer Adding Acid To Buffer Calculation Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution. Hydrogen ions combine with the ethanoate ions to. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Buffers allow chemists to maintain a specific ph. Adding Acid To Buffer Calculation.
From www.youtube.com
Adding strong acid to a buffer (Ch 2, 27) YouTube Adding Acid To Buffer Calculation Adding an acid to this buffer solution. Calculation of the ph of a buffer solution after addition of a small amount of acid. Calculate the ph of a buffer before and after the addition of added acid or. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Hydrogen ions. Adding Acid To Buffer Calculation.
From www.brainkart.com
Buffers Adding Acid To Buffer Calculation Calculation of the ph of a buffer solution after addition of a small amount of acid. Calculate the ph of a buffer before and after the addition of added acid or. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution the buffer. Adding Acid To Buffer Calculation.
From www.youtube.com
Find the pH of a Buffer after adding NaOH YouTube Adding Acid To Buffer Calculation Calculation of the ph of a buffer solution after addition of a small amount of acid. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Buffers allow chemists to maintain a specific ph range for a reaction. Calculate the ph of a buffer before and after the addition of added acid or.. Adding Acid To Buffer Calculation.
From www.slideshare.net
2012 topic 18 2 buffer solutions Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Buffers allow chemists to maintain a specific ph range for a reaction. The buffer solution must remove most of the new hydrogen ions otherwise the. Adding Acid To Buffer Calculation.
From www.youtube.com
Calculating the pH of a buffer YouTube Adding Acid To Buffer Calculation Buffers allow chemists to maintain a specific ph range for a reaction. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Hydrogen ions combine with the ethanoate ions. Adding Acid To Buffer Calculation.
From www.youtube.com
AP chemistry Adding Strong Acid to Buffer problem YouTube Adding Acid To Buffer Calculation Calculation of the ph of a buffer solution after addition of a small amount of acid. Hydrogen ions combine with the ethanoate ions to. Calculate the ph of a buffer before and after the addition of added acid or. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. When. Adding Acid To Buffer Calculation.
From www.youtube.com
Find the pH of a Buffer after Adding HCl YouTube Adding Acid To Buffer Calculation Calculation of the ph of a buffer solution after addition of a small amount of acid. Adding an acid to this buffer solution. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen. Adding Acid To Buffer Calculation.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube Adding Acid To Buffer Calculation Calculation of the ph of a buffer solution after addition of a small amount of acid. Adding an acid to this buffer solution. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Buffers allow chemists to maintain a specific ph range for a reaction. The. Adding Acid To Buffer Calculation.
From chemguru.sg
Calculate pH of Buffer Solution Adding Acid To Buffer Calculation When a strong acid (h 3 o +) is added to a buffer solution the. Hydrogen ions combine with the ethanoate ions to. Calculation of the ph of a buffer solution after addition of a small amount of acid. Adding an acid to this buffer solution. The buffer solution must remove most of the new hydrogen ions otherwise the ph. Adding Acid To Buffer Calculation.
From www.youtube.com
8 Buffer Calculations 3 YouTube Adding Acid To Buffer Calculation Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. When a strong acid (h 3 o +) is. Adding Acid To Buffer Calculation.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. When a strong acid (h 3 o +) is added to a buffer solution the. Calculation of the ph of a buffer solution after addition. Adding Acid To Buffer Calculation.
From bestonlinecollegesdegrees.com
Tris Buffer Recipe Calculator Besto Blog Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Buffers allow chemists to maintain a specific ph range for a. Adding Acid To Buffer Calculation.
From study.com
AcidBase Buffers Calculating the pH of a Buffered Solution Video Adding Acid To Buffer Calculation Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Buffers allow chemists to maintain a specific ph range for a reaction. Calculate the ph of a buffer before. Adding Acid To Buffer Calculation.
From studylib.net
Buffer Calculations for Polyprotic Acids Adding Acid To Buffer Calculation Buffers allow chemists to maintain a specific ph range for a reaction. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. Calculate the ph of a buffer before and after the addition of added acid or. When a. Adding Acid To Buffer Calculation.
From www.youtube.com
Adding an acid to buffer solution change in pH calculation (A Level Adding Acid To Buffer Calculation The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Calculation of the ph of a buffer solution after addition of a small amount of acid.. Adding Acid To Buffer Calculation.
From www.youtube.com
Acids and Bases Buffer Calculation Past Paper Exam Question Adding Acid To Buffer Calculation Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Buffers allow chemists to maintain a specific ph range for a reaction. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution.. Adding Acid To Buffer Calculation.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Adding Acid To Buffer Calculation Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Adding an acid to this buffer solution. Buffers allow chemists to maintain a specific ph range. Adding Acid To Buffer Calculation.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Adding an acid to this buffer solution. Buffers allow chemists to maintain a specific ph range for a reaction. The buffer solution must remove most of the new. Adding Acid To Buffer Calculation.
From www.youtube.com
How to Calculate PH of Buffer Solution YouTube Adding Acid To Buffer Calculation Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Hydrogen ions combine with the ethanoate ions to. Calculation of the ph of a buffer solution after addition of a small amount of acid. Adding an acid to this buffer solution. Buffers allow chemists to maintain a specific ph range. Adding Acid To Buffer Calculation.
From www.chegg.com
Solved Weak Acid solution used to prepare buffers a and b Adding Acid To Buffer Calculation Calculation of the ph of a buffer solution after addition of a small amount of acid. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution. Buffers allow chemists to maintain a specific ph range. Adding Acid To Buffer Calculation.
From www.youtube.com
pH of buffer solutions the HendersonHasselbalch equation. YouTube Adding Acid To Buffer Calculation Adding an acid to this buffer solution. When a strong acid (h 3 o +) is added to a buffer solution the. Calculate the ph of a buffer before and after the addition of added acid or. Adding an acid to this buffer solution the buffer solution must remove most of the new hydrogen ions otherwise the ph would drop. Adding Acid To Buffer Calculation.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Adding Acid To Buffer Calculation Buffers allow chemists to maintain a specific ph range for a reaction. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculate the ph of a buffer before and after the addition of added acid or. When a strong acid (h 3 o +) is added to a buffer solution the. Hydrogen. Adding Acid To Buffer Calculation.
From www.youtube.com
pH of Buffer Solution (Example) YouTube Adding Acid To Buffer Calculation Hydrogen ions combine with the ethanoate ions to. Adding an acid to this buffer solution. Calculate the ph of a buffer before and after the addition of added acid or. Buffers allow chemists to maintain a specific ph range for a reaction. Calculation of the ph of a buffer solution after addition of a small amount of acid. The buffer. Adding Acid To Buffer Calculation.
From www.youtube.com
How to calculate the pH of a buffer solution YouTube Adding Acid To Buffer Calculation Adding an acid to this buffer solution. When a strong acid (h 3 o +) is added to a buffer solution the. Calculate the ph of a buffer before and after the addition of added acid or. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Hydrogen ions combine with the ethanoate. Adding Acid To Buffer Calculation.