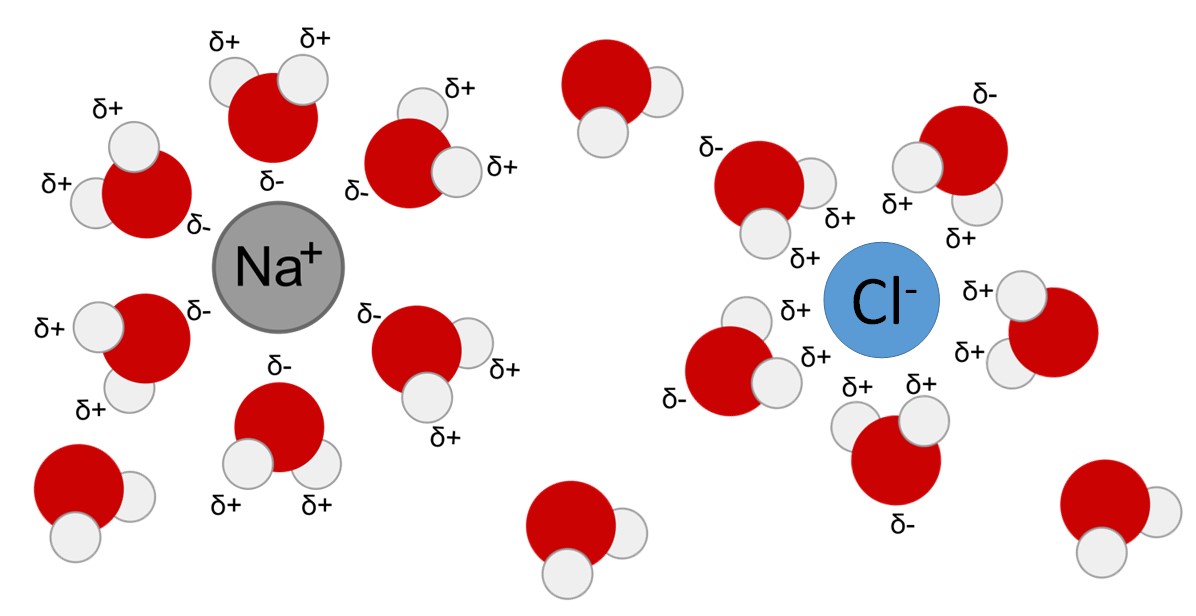
When Table Salt Dissolves In Water The Sodium Ions Are Attracted To . When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. 1 identify the components of table salt (nacl). When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. When nacl dissolves in water, it dissociates into. 2 the na+ ions are. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent
from rwu.pressbooks.pub
When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. 2 the na+ ions are. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When nacl dissolves in water, it dissociates into. 1 identify the components of table salt (nacl). We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water.
Chapter 2. The Chemical Context of Life Introduction to Molecular and
When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. When nacl dissolves in water, it dissociates into. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. 1 identify the components of table salt (nacl). When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. 2 the na+ ions are. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules.
From wou.edu
CH104 Chapter 7 Solutions Chemistry When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. 1 identify the components of table salt (nacl). When salt. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From shaunmwilliams.com
Chapter 11 Presentation When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. 1 identify the components of table salt (nacl). 2 the na+ ions are. When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From wou.edu
CH150 Chapter 7 Solutions Chemistry When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.baristahustle.com
TWC 0.02 How Does Water Dissolve Mineral Salts? Barista Hustle When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When table salt is dissolved in water, the salt acts as _____ and the water is the solvent We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When table salt is dissolved in water, the salt acts as _____ and the water is the. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.freethought-forum.com
On the Anatomy and Physiology of Sight Freethought Forum When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 2 the na+ ions are. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent 1 identify the components of table salt (nacl). We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When salt is mixed with water, the salt. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From courses.lumenlearning.com
Compounds Essential to Human Functioning Anatomy and When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. 2 the na+ ions are. When salt is mixed with water, the salt dissolves because the covalent bonds of water. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.youtube.com
How Salt Dissolves in Water? YouTube When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. 2 the na+ ions are. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When salt is added to water, the positive and negative ions are. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.expii.com
Good Solvent (Water) — Properties & Examples Expii When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. 1 identify the components of table salt (nacl). When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.youtube.com
How Water Dissolves Salt YouTube When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 1 identify the components of table salt (nacl). We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When salt is added to water, the positive and negative. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From philschatz.com
Electrolytes · Chemistry When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. 1 identify the components of table salt (nacl). We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. 1 identify the components of table salt (nacl). When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From brainly.com
The graph shows the solubility of several different salts in water When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When table salt is dissolved in water, the salt acts as _____ and the water is the solvent 1 identify the components of table salt (nacl). When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. We will first examine the process that occurs when. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 1 identify the components of table salt (nacl). When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. 2 the na+ ions are. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When table salt is. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. 1 identify the components of table salt (nacl). When salt is mixed with water, the salt dissolves because the covalent. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.youtube.com
table salt dissolves in water YouTube When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 2 the na+ ions are. When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When table salt is dissolved in water, the salt acts as _____. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.chegg.com
Solved Reaction 1 Solid sodium hydroxide dissolves in water When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 2 the na+ ions are. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When salt is added to water, the positive and negative ions are attracted to the oppositely. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From rwu.pressbooks.pub
Chapter 2. The Chemical Context of Life Introduction to Molecular and When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. 1 identify the components of table salt (nacl). When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent We. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When nacl dissolves in water, it dissociates into. When salt is added to water,. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From userdatarheumatics.z21.web.core.windows.net
Chemical Equation For Salt Dissolved In Water When Table Salt Dissolves In Water The Sodium Ions Are Attracted To We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When table salt is dissolved in water, the salt acts as _____ and the water is the solvent 2 the na+ ions are. When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. When nacl dissolves in water, it dissociates into. 1 identify the components of table salt. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. 2 the na+ ions are. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. We will first examine the process that occurs when. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记6.3.3 Reactions of Ions in Aqueous When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 2 the na+ ions are. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When nacl dissolves in water, it dissociates into. 1 identify the components. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The When Table Salt Dissolves In Water The Sodium Ions Are Attracted To We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When nacl dissolves in water, it dissociates into. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. When salt is added to water,. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 2 the na+ ions are. When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. We will first examine the process that occurs. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From brainly.in
When NaCl dissolves in water, aqueous Na+ and Cl− ions result. The When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule. 1 identify the components of table salt (nacl). When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When nacl dissolves in water, it dissociates into. We will first examine. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From www.youtube.com
table salt dissolves in water YouTube When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 2 the na+ ions are. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When table salt is dissolved in water, the salt. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From exogqaefj.blob.core.windows.net
Copper Chloride Water Solubility at Sandra Barrera blog When Table Salt Dissolves In Water The Sodium Ions Are Attracted To 1 identify the components of table salt (nacl). We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When nacl dissolves in water, it dissociates into. 2 the na+ ions are. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From hxegxgchx.blob.core.windows.net
Table Salt Chemical Reaction at Fergus blog When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. 2 the na+ ions are. 1 identify the components of table salt (nacl). When salt is added to water, the positive and negative ions are attracted to the oppositely charged ends of the water molecule.. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From mavink.com
Ion Induced Dipole Forces When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When nacl dissolves in water, it dissociates into. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent We will first examine the process that occurs when. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From users.highland.edu
Aqueous Solutions When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent We will first examine the process that occurs when an ionic compound such as table salt (sodium. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.
From sphweb.bumc.bu.edu
Chemical Elements Atoms When Table Salt Dissolves In Water The Sodium Ions Are Attracted To When table salt is dissolved in water, the salt acts as _____ and the water is the solvent When nacl dissolves in water, it dissociates into. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. 2 the na+ ions are. 1 identify the components of table salt (nacl).. When Table Salt Dissolves In Water The Sodium Ions Are Attracted To.