How Does Calcium And Potassium React With Water . More reactive metals displace less reactive metals from. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. It moves around very quickly on the surface of the water. calcium reacts readily with water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Calcium + water calcium hydroxide + hydrogen. when potassium is added to water, the metal melts and floats. ii write equation for the reaction of calcium and potassium with water. 9 rows the reactivity series ranks metals by how readily they react. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:
from iu.pressbooks.pub
all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. ii write equation for the reaction of calcium and potassium with water. 9 rows the reactivity series ranks metals by how readily they react. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: More reactive metals displace less reactive metals from. Calcium + water calcium hydroxide + hydrogen. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. It moves around very quickly on the surface of the water. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. calcium reacts readily with water.
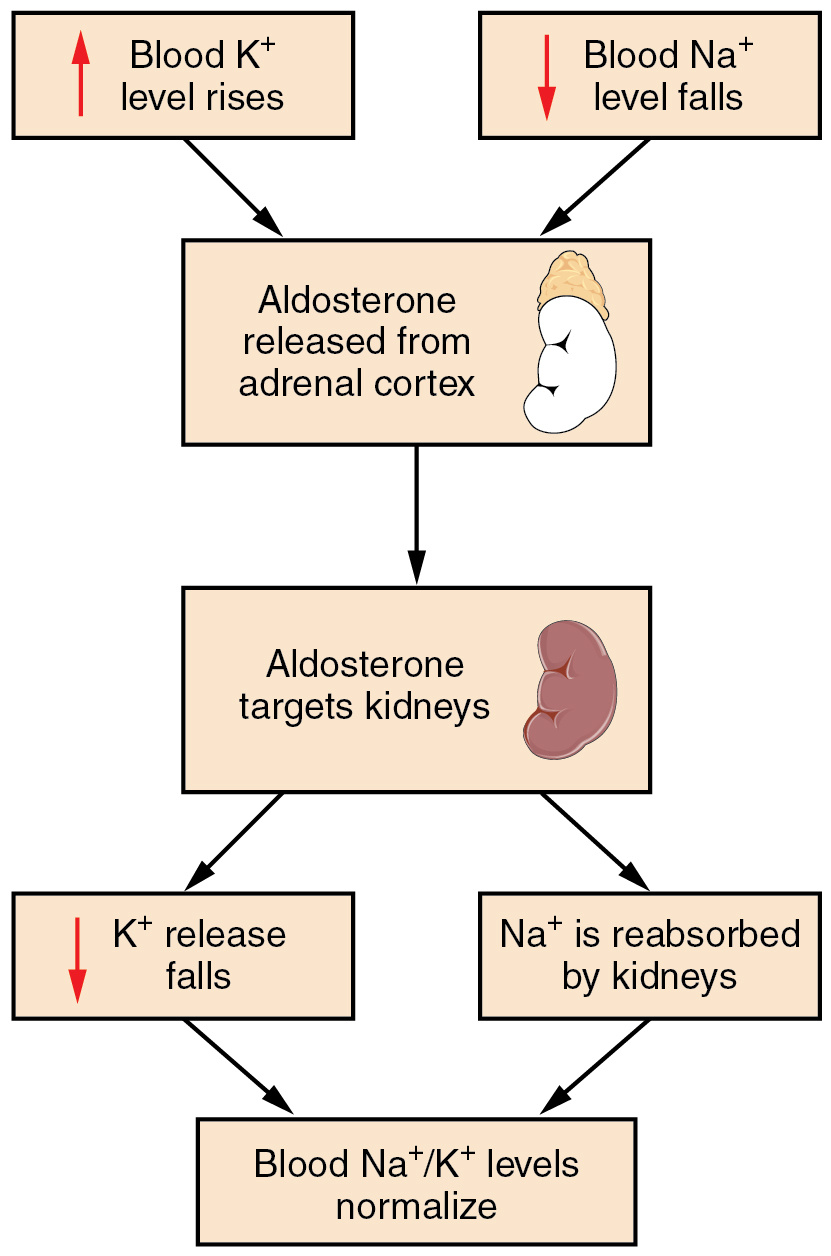
Potassium and calcium homeostasis Basic Human Physiology
How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. ii write equation for the reaction of calcium and potassium with water. Calcium + water calcium hydroxide + hydrogen. when potassium is added to water, the metal melts and floats. More reactive metals displace less reactive metals from. It moves around very quickly on the surface of the water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: 9 rows the reactivity series ranks metals by how readily they react. calcium reacts readily with water. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts.
From mammothmemory.net
Potassium is very reactive when introduced to water How Does Calcium And Potassium React With Water ii write equation for the reaction of calcium and potassium with water. It moves around very quickly on the surface of the water. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium,. How Does Calcium And Potassium React With Water.
From www.youtube.com
Potassium in water reaction MVI 1348 YouTube How Does Calcium And Potassium React With Water when potassium is added to water, the metal melts and floats. ii write equation for the reaction of calcium and potassium with water. Calcium + water calcium hydroxide + hydrogen. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. sodium (na), potassium (k), and calcium (ca) are the. How Does Calcium And Potassium React With Water.
From www.alamy.com
Calcium reacting with water Stock Photo Alamy How Does Calcium And Potassium React With Water Calcium + water calcium hydroxide + hydrogen. More reactive metals displace less reactive metals from. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. when potassium is added to water, the metal melts and floats. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or. How Does Calcium And Potassium React With Water.
From fphoto.photoshelter.com
science chemistry exothermic reaction potassium water Fundamental How Does Calcium And Potassium React With Water sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. It moves around very quickly on the surface of the water. when potassium is added to water, the metal melts and floats. 9 rows the reactivity series ranks metals by how readily they react. calcium reacts readily with water. More. How Does Calcium And Potassium React With Water.
From iu.pressbooks.pub
Potassium and calcium homeostasis Basic Human Physiology How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. It moves around very quickly on the surface of the water. when potassium is added to water, the metal melts and floats. Calcium + water calcium hydroxide +. How Does Calcium And Potassium React With Water.
From www.youtube.com
Reaction between Calcium and Water YouTube How Does Calcium And Potassium React With Water all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. ii write equation for the reaction of calcium and potassium with water. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. when potassium is added to water, the metal melts and. How Does Calcium And Potassium React With Water.
From www.emporiumhydroponics.com
Potassium versus Calcium How Does Calcium And Potassium React With Water calcium reacts readily with water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. ii write equation for the reaction of calcium and potassium with water. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. More reactive metals displace. How Does Calcium And Potassium React With Water.
From klaixelt.blogspot.com
Potassium Reaction With Water potassium water exothermic reaction How Does Calcium And Potassium React With Water the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. More reactive metals displace less reactive metals from. It moves around very quickly on the surface of the water. ii. How Does Calcium And Potassium React With Water.
From fphoto.photoshelter.com
science chemistry exothermic reaction potassium water Fundamental How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. sodium (na), potassium (k), and calcium (ca). How Does Calcium And Potassium React With Water.
From www.youtube.com
Calcium is added to water YouTube How Does Calcium And Potassium React With Water More reactive metals displace less reactive metals from. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Calcium + water calcium hydroxide + hydrogen. 9 rows the reactivity series ranks metals by how readily they react. It moves around very quickly on the surface of the water. the. How Does Calcium And Potassium React With Water.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation, free How Does Calcium And Potassium React With Water calcium reacts readily with water. It moves around very quickly on the surface of the water. 9 rows the reactivity series ranks metals by how readily they react. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. More reactive metals displace less reactive metals from. Calcium + water calcium hydroxide. How Does Calcium And Potassium React With Water.
From www.sciencephoto.com
Potassium reacting with water Stock Image C024/5715 Science Photo How Does Calcium And Potassium React With Water Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. 9 rows the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from. calcium reacts readily with water. the general reaction of calcium, strontium, and barium with water is represented below, where. How Does Calcium And Potassium React With Water.
From www.numerade.com
SOLVED In what ways is the reaction between calcium and water How Does Calcium And Potassium React With Water More reactive metals displace less reactive metals from. 9 rows the reactivity series ranks metals by how readily they react. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. calcium reacts readily with water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. How Does Calcium And Potassium React With Water.
From fphoto.photoshelter.com
potassium water exothermic reaction science Fundamental Photographs How Does Calcium And Potassium React With Water calcium reacts readily with water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: when potassium is added to water, the metal melts and floats. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. sodium. How Does Calcium And Potassium React With Water.
From www.sciencephoto.com
Potassium reacting with water Stock Image C015/0432 Science Photo How Does Calcium And Potassium React With Water the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: calcium reacts readily with water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. ii write equation for the reaction of calcium and potassium with water. More. How Does Calcium And Potassium React With Water.
From www.sciencephoto.com
Potassium Reacts with Water Stock Image C039/1186 Science Photo How Does Calcium And Potassium React With Water ii write equation for the reaction of calcium and potassium with water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: sodium (na), potassium (k), and calcium. How Does Calcium And Potassium React With Water.
From sciencephotogallery.com
Potassium Reacting With Water by Science Photo Library How Does Calcium And Potassium React With Water ii write equation for the reaction of calcium and potassium with water. calcium reacts readily with water. Calcium + water calcium hydroxide + hydrogen. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it. How Does Calcium And Potassium React With Water.
From telgurus.co.uk
A balanced chemical equation for a reaction between potassium and water How Does Calcium And Potassium React With Water It moves around very quickly on the surface of the water. when potassium is added to water, the metal melts and floats. Calcium + water calcium hydroxide + hydrogen. More reactive metals displace less reactive metals from. calcium reacts readily with water. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) ,. How Does Calcium And Potassium React With Water.
From www.slideserve.com
PPT Ch 17. J.C. Rowe PowerPoint Presentation ID3099881 How Does Calcium And Potassium React With Water Calcium + water calcium hydroxide + hydrogen. 9 rows the reactivity series ranks metals by how readily they react. ii write equation for the reaction of calcium and potassium with water. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. It moves around very quickly on the surface of. How Does Calcium And Potassium React With Water.
From www.youtube.com
Reaction of Potassium with Water Chemistry Practicals YouTube How Does Calcium And Potassium React With Water ii write equation for the reaction of calcium and potassium with water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. Ca (s) + 2h 2 o (l) ca (oh). How Does Calcium And Potassium React With Water.
From www.slideserve.com
PPT Chemical Equations & Reactions PowerPoint Presentation, free How Does Calcium And Potassium React With Water It moves around very quickly on the surface of the water. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: when potassium is added to water, the metal melts. How Does Calcium And Potassium React With Water.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW How Does Calcium And Potassium React With Water It moves around very quickly on the surface of the water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: ii write equation for the reaction of calcium and potassium with water. when potassium is added to water, the metal melts and floats. all of. How Does Calcium And Potassium React With Water.
From klaixelt.blogspot.com
Potassium Reaction With Water potassium water exothermic reaction How Does Calcium And Potassium React With Water all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. More reactive metals displace less reactive metals from. the general reaction of calcium, strontium, and barium with water is represented below, where. How Does Calcium And Potassium React With Water.
From brainly.in
Word equation for reaction between calcium and water Brainly.in How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. Calcium + water calcium hydroxide + hydrogen. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: calcium reacts readily with water. sodium (na), potassium (k), and calcium (ca) are the metal which reacts. How Does Calcium And Potassium React With Water.
From www.youtube.com
The Reaction of Calcium Metal and Water YouTube How Does Calcium And Potassium React With Water More reactive metals displace less reactive metals from. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. It moves around very quickly on the surface of the water. ii write equation for. How Does Calcium And Potassium React With Water.
From www.youtube.com
Reaction of Potassium with water YouTube How Does Calcium And Potassium React With Water ii write equation for the reaction of calcium and potassium with water. calcium reacts readily with water. Calcium + water calcium hydroxide + hydrogen. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. 9 rows the reactivity series ranks metals by how readily they react. It moves around very. How Does Calcium And Potassium React With Water.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. when potassium is added to water, the metal melts and floats. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. ii write equation for the reaction of calcium and potassium with water. calcium reacts readily with. How Does Calcium And Potassium React With Water.
From www.sciencephoto.com
Calcium Reacting with Water Stock Image C027/9558 Science Photo Library How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. when potassium is added to water, the metal melts and floats. Calcium + water calcium hydroxide + hydrogen. calcium reacts readily with water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: . How Does Calcium And Potassium React With Water.
From fphoto.photoshelter.com
potassium water exothermic reaction science Fundamental Photographs How Does Calcium And Potassium React With Water It moves around very quickly on the surface of the water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. when potassium is added to water, the metal melts and floats.. How Does Calcium And Potassium React With Water.
From www.youtube.com
Write equations for the reactions of Calcium and potassium with water How Does Calcium And Potassium React With Water It moves around very quickly on the surface of the water. ii write equation for the reaction of calcium and potassium with water. More reactive metals displace less reactive metals from. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. when potassium is added to water, the metal melts and. How Does Calcium And Potassium React With Water.
From www.slideserve.com
PPT The Reaction of Calcium with Water PowerPoint Presentation, free How Does Calcium And Potassium React With Water Calcium + water calcium hydroxide + hydrogen. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium: calcium reacts readily with water. sodium (na), potassium (k), and calcium (ca). How Does Calcium And Potassium React With Water.
From www.sciencephoto.com
Potassium reacting with water Stock Image A500/0376 Science Photo How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. ii write equation for the reaction of calcium and potassium with water. when potassium is added to water, the metal melts and floats. More reactive metals displace less reactive metals from. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously. How Does Calcium And Potassium React With Water.
From pixels.com
Calcium Reacting With Water Photograph by Andrew Lambert Photography How Does Calcium And Potassium React With Water 9 rows the reactivity series ranks metals by how readily they react. It moves around very quickly on the surface of the water. sodium (na), potassium (k), and calcium (ca) are the metal which reacts readily with cold water. all of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold.. How Does Calcium And Potassium React With Water.
From www.ncbi.nlm.nih.gov
[Figure, Figure 2 mechanism of calcium...] StatPearls NCBI Bookshelf How Does Calcium And Potassium React With Water Calcium + water calcium hydroxide + hydrogen. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. 9 rows the reactivity series ranks metals by how readily they react. when potassium is added to water, the metal melts and floats. More reactive metals displace less reactive metals from. the. How Does Calcium And Potassium React With Water.
From www.youtube.com
Elemental Potassium Reaction With Water YouTube How Does Calcium And Potassium React With Water when potassium is added to water, the metal melts and floats. calcium reacts readily with water. 9 rows the reactivity series ranks metals by how readily they react. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. sodium (na), potassium (k), and calcium (ca) are the metal. How Does Calcium And Potassium React With Water.