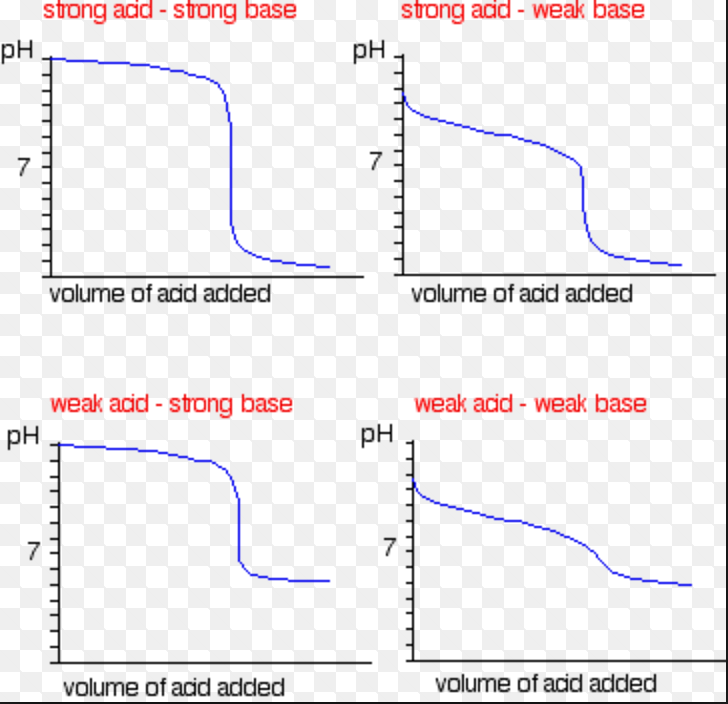
Ph Titration Titrant . 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: These curves are useful in selecting. The equivalence point of a titration. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. There are two basic types of acid base titrations, indicator and potentiometric. Compute sample ph at important stages of a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. We also will learn how to sketch a good. Sorting out some confusing terms. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes.
from classnotes.org.in
Sorting out some confusing terms. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: These curves are useful in selecting. Compute sample ph at important stages of a titration. We also will learn how to sketch a good. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The equivalence point of a titration.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
Ph Titration Titrant We also will learn how to sketch a good. We also will learn how to sketch a good. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The equivalence point of a titration. Compute sample ph at important stages of a titration. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Sorting out some confusing terms. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. These curves are useful in selecting. There are two basic types of acid base titrations, indicator and potentiometric. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Ph Titration Titrant As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The equivalence point of a titration. Sorting out some confusing terms. We also will learn how to sketch a good. Calculate the ph for the weak. Ph Titration Titrant.
From www.youtube.com
pH Titrations Calculations YouTube Ph Titration Titrant There are two basic types of acid base titrations, indicator and potentiometric. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. In this section we will learn how to calculate. Ph Titration Titrant.
From www.youtube.com
titration curve pH calculations YouTube Ph Titration Titrant These curves are useful in selecting. Sorting out some confusing terms. The equivalence point of a titration. Compute sample ph at important stages of a titration. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. We also will learn how to sketch a good. In an indicator based titration you add another chemical that changes color at the ph equal. Ph Titration Titrant.
From www.slideserve.com
PPT Titration and pH Curves. PowerPoint Presentation, free download Ph Titration Titrant Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: These curves are useful in selecting. Compute sample ph at important stages of a titration. In this section we will learn how to calculate the ph of an analyte solution. Ph Titration Titrant.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Ph Titration Titrant Sorting out some confusing terms. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Compute sample ph at important stages of a titration. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are. Ph Titration Titrant.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Ph Titration Titrant The equivalence point of a titration. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). Ph Titration Titrant.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Ph Titration Titrant The equivalence point of a titration. Sorting out some confusing terms. These curves are useful in selecting. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. In this section we will learn how to calculate. Ph Titration Titrant.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Ph Titration Titrant 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: These curves are useful in selecting. In an indicator based titration you add another chemical that changes color at the ph. Ph Titration Titrant.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Ph Titration Titrant 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. We also will learn how to sketch a good. Compute sample ph at important stages of a titration. Sorting out some confusing terms. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of. Ph Titration Titrant.
From saylordotorg.github.io
AcidBase Titrations Ph Titration Titrant As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. The following example exercise demonstrates the computation of ph for. Ph Titration Titrant.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Ph Titration Titrant The equivalence point of a titration. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: We also will learn how to sketch a good. There are two basic types of acid base titrations, indicator and potentiometric. The following example. Ph Titration Titrant.
From morioh.com
Acid Base Titration Curves PH Calculations Ph Titration Titrant In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: These. Ph Titration Titrant.
From studylib.net
Titrations of Acids and Bases Ph Titration Titrant Sorting out some confusing terms. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be.. Ph Titration Titrant.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Ph Titration Titrant These curves are useful in selecting. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. There are two basic types of acid base titrations, indicator and. Ph Titration Titrant.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Ph Titration Titrant We also will learn how to sketch a good. The equivalence point of a titration. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq). Ph Titration Titrant.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Ph Titration Titrant Sorting out some confusing terms. Compute sample ph at important stages of a titration. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: There are two basic types of acid. Ph Titration Titrant.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Ph Titration Titrant In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. These curves are useful in selecting. There are two basic types of acid base titrations, indicator and potentiometric. Calculate the ph for the weak acid/strong base titration between 50.0 ml of. Ph Titration Titrant.
From roqed.com
Titration (pH) curves ROQED Ph Titration Titrant Sorting out some confusing terms. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. We also will learn how to sketch a good. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. In an indicator based titration you add another chemical that. Ph Titration Titrant.
From byjus.com
The graph of pH during the titration of NaOH and HCl Ph Titration Titrant As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Compute sample ph at important stages of a titration. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. There are two basic types of acid base titrations, indicator. Ph Titration Titrant.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Ph Titration Titrant The equivalence point of a titration. We also will learn how to sketch a good. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. In this section we will learn how to calculate the ph of an analyte solution throughout. Ph Titration Titrant.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Ph Titration Titrant There are two basic types of acid base titrations, indicator and potentiometric. The equivalence point of a titration. Compute sample ph at important stages of a titration. These curves are useful in selecting. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. The following example exercise. Ph Titration Titrant.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Ph Titration Titrant The equivalence point of a titration. Sorting out some confusing terms. These curves are useful in selecting. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. We also will learn how. Ph Titration Titrant.
From ck12.org
Titration CK12 Foundation Ph Titration Titrant As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Compute sample ph at important stages of a titration. Calculate the ph for the weak acid/strong base titration between. Ph Titration Titrant.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Ph Titration Titrant In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: We. Ph Titration Titrant.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Ph Titration Titrant As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Sorting out some confusing terms. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: In this section we will learn how to calculate the ph. Ph Titration Titrant.
From slideplayer.com
Titrations pH Titrant volume, mL ppt download Ph Titration Titrant In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The equivalence point of a titration. Sorting out some confusing terms. Compute sample ph at important stages of a titration. As seen in the chapter on the stoichiometry of chemical reactions,. Ph Titration Titrant.
From www.measurenet-tech.com
pH Electrode for pH titrations Ph Titration Titrant There are two basic types of acid base titrations, indicator and potentiometric. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. We also will learn how to sketch a good. These curves are useful in selecting. Calculate the ph for the weak acid/strong base titration between 50.0 ml of. Ph Titration Titrant.
From saylordotorg.github.io
AcidBase Titrations Ph Titration Titrant There are two basic types of acid base titrations, indicator and potentiometric. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. The equivalence point of a titration. Calculate the ph for the weak. Ph Titration Titrant.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Ph Titration Titrant We also will learn how to sketch a good. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. There are two basic types of acid base titrations, indicator and potentiometric. The equivalence point of a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Compute sample ph at important stages of a titration. Sorting. Ph Titration Titrant.
From saylordotorg.github.io
AcidBase Titrations Ph Titration Titrant These curves are useful in selecting. Sorting out some confusing terms. There are two basic types of acid base titrations, indicator and potentiometric. 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric. Ph Titration Titrant.
From mmerevise.co.uk
pH Curves Questions and Revision MME Ph Titration Titrant We also will learn how to sketch a good. The equivalence point of a titration. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. Sorting out some confusing terms. These curves are useful in selecting. There are two basic types. Ph Titration Titrant.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Ph Titration Titrant In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The equivalence point of a titration. Compute sample ph at important stages of a titration. These curves are useful in selecting. Calculate the ph for the weak acid/strong base titration between. Ph Titration Titrant.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube Ph Titration Titrant These curves are useful in selecting. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Compute sample ph at important stages of a titration. The equivalence point of a titration. Sorting out some confusing terms. 0.00 ml, 15.0 ml, 25.0 ml, and. Ph Titration Titrant.
From www.researchgate.net
The Graph of pH vs Volume of titrant for Potentiometric Titration of Ph Titration Titrant 0.00 ml, 15.0 ml, 25.0 ml, and 30.0 ml. These curves are useful in selecting. Compute sample ph at important stages of a titration. Sorting out some confusing terms. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed volumes of added base: The. Ph Titration Titrant.
From slideplayer.com
Titrations pH Titrant volume, mL ppt download Ph Titration Titrant Compute sample ph at important stages of a titration. These curves are useful in selecting. The equivalence point of a titration. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. There are two basic types of acid base titrations, indicator and potentiometric. 0.00 ml, 15.0 ml, 25.0 ml, and. Ph Titration Titrant.