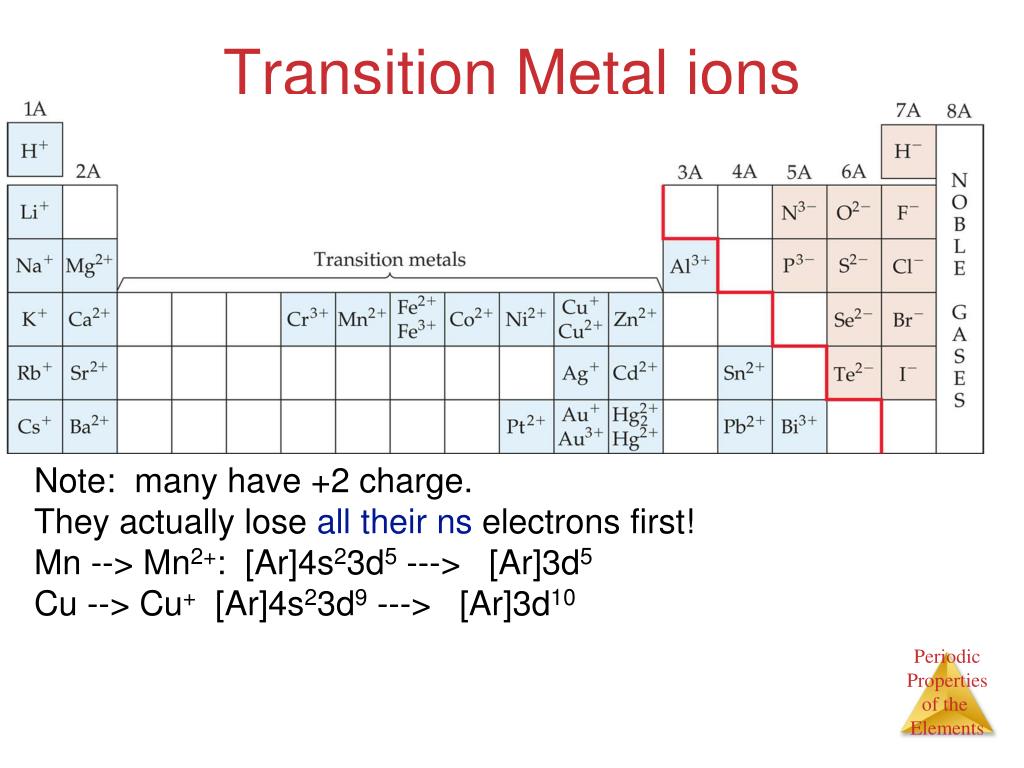
Are Transition Metals Ionic . Therefore, these metal oxides are ionic. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. As a result, transition metals are able lose inner shell electrons, in addition to their valence electrons. The field of complexes is. Although the next higher \(s\) orbitals are. Embedded video, no tabs, this description appears on section page: Transition metals in low oxidation states have lower electronegativity values than oxygen; These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. Transition metals in low oxidation states have lower electronegativity values than oxygen; You will find some of this covered quite briefly on this page with. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. Therefore, these metal oxides are ionic.
from www.slideserve.com
Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Although the next higher \(s\) orbitals are. The field of complexes is. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Embedded video, no tabs, this description appears on section page: Therefore, these metal oxides are ionic. You will find some of this covered quite briefly on this page with. Transition metals in low oxidation states have lower electronegativity values than oxygen;
PPT Chapter 7 Periodic Properties of the Elements PowerPoint
Are Transition Metals Ionic Therefore, these metal oxides are ionic. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. The field of complexes is. Although the next higher \(s\) orbitals are. Therefore, these metal oxides are ionic. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Therefore, these metal oxides are ionic. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. As a result, transition metals are able lose inner shell electrons, in addition to their valence electrons. Embedded video, no tabs, this description appears on section page: Transition metals in low oxidation states have lower electronegativity values than oxygen; Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. Transition metals in low oxidation states have lower electronegativity values than oxygen; You will find some of this covered quite briefly on this page with. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity.
From www.youtube.com
Naming Ionic Compounds with Transition Metals Introduction YouTube Are Transition Metals Ionic The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. As a result, transition metals are able lose inner shell electrons, in addition to their valence electrons. The field of complexes is. Therefore, these metal oxides are ionic. Although the next higher \(s\) orbitals. Are Transition Metals Ionic.
From www.slideserve.com
PPT Polyatomic ions & Naming ionic Compounds PowerPoint Presentation Are Transition Metals Ionic These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. Therefore, these metal oxides are ionic. Embedded video, no tabs, this description appears on section page: Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Although the next higher \(s\) orbitals are. The formation of complexes. Are Transition Metals Ionic.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID4961184 Are Transition Metals Ionic Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. The field of complexes is. You will find some of this covered quite briefly on this page with. Transition metals in low oxidation states have lower electronegativity values than oxygen; As a result, transition metals are able. Are Transition Metals Ionic.
From www.youtube.com
Transition Metals in Ionic Formulas YouTube Are Transition Metals Ionic Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. Transition metals in low oxidation states have lower electronegativity values than oxygen; Therefore, these metal oxides are ionic. You will find some of this covered quite briefly on this page with. Embedded video, no tabs, this description. Are Transition Metals Ionic.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Are Transition Metals Ionic You will find some of this covered quite briefly on this page with. Therefore, these metal oxides are ionic. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. Transition metals. Are Transition Metals Ionic.
From www.youtube.com
How To Name Ionic Compounds With Transition Metals YouTube Are Transition Metals Ionic Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Transition metals in low oxidation states have lower electronegativity values than oxygen; The field of complexes. Are Transition Metals Ionic.
From www.slideserve.com
PPT Ionic Compounds Containing Transition Metals (p. 55) PowerPoint Are Transition Metals Ionic Although the next higher \(s\) orbitals are. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Therefore, these metal oxides are ionic. Embedded video, no tabs, this description appears on section page: These include variable. Are Transition Metals Ionic.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Are Transition Metals Ionic Transition metals in low oxidation states have lower electronegativity values than oxygen; Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. These include variable oxidation. Are Transition Metals Ionic.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID1931344 Are Transition Metals Ionic Embedded video, no tabs, this description appears on section page: Therefore, these metal oxides are ionic. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Therefore, these metal. Are Transition Metals Ionic.
From byjus.com
Transition Elements What Are The Causes Of Ionisation Enthalpy Are Transition Metals Ionic The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Although the next higher \(s\) orbitals are. Therefore, these metal oxides are ionic. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. You will find some of this. Are Transition Metals Ionic.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download Are Transition Metals Ionic Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Although the next higher \(s\) orbitals are. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the. Are Transition Metals Ionic.
From www.youtube.com
Ionic Compounds and Bonding Part 06 Transition Metal Nomenclature Are Transition Metals Ionic Therefore, these metal oxides are ionic. Embedded video, no tabs, this description appears on section page: The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Therefore, these metal oxides are ionic. Although the next higher \(s\) orbitals are. The formation of complexes causes the d orbitals to split into two energy sublevels, which. Are Transition Metals Ionic.
From www.slideserve.com
PPT Ionic Compounds Containing Multivalent Ions PowerPoint Are Transition Metals Ionic Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Although the next higher \(s\) orbitals are. You will find some of this covered quite briefly on this page with. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or. Are Transition Metals Ionic.
From www.slideserve.com
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free Are Transition Metals Ionic These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Embedded video, no tabs, this description appears on section page: The field of complexes is. Transition metals in low oxidation states have lower electronegativity values than oxygen;. Are Transition Metals Ionic.
From www.youtube.com
How to Name Ionic Compounds with Transition Metals YouTube Are Transition Metals Ionic Transition metals in low oxidation states have lower electronegativity values than oxygen; The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. The formation of complexes causes the d orbitals to. Are Transition Metals Ionic.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Are Transition Metals Ionic Therefore, these metal oxides are ionic. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Embedded video, no tabs, this description appears on section page: Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells.. Are Transition Metals Ionic.
From www.slideserve.com
PPT Ions and Ionic Compound PowerPoint Presentation, free download Are Transition Metals Ionic Transition metals in low oxidation states have lower electronegativity values than oxygen; Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Although the next higher \(s\) orbitals are. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. The formation of complexes causes the d orbitals. Are Transition Metals Ionic.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download Are Transition Metals Ionic Transition metals in low oxidation states have lower electronegativity values than oxygen; The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Therefore, these metal oxides are ionic. Although the next higher \(s\) orbitals are. Therefore,. Are Transition Metals Ionic.
From www.slideserve.com
PPT Names and Formulas Ionic Compounds Binary Main Group M & NM Using Are Transition Metals Ionic The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. The field of complexes is. Transition metals in low oxidation states have lower electronegativity values than oxygen; Therefore, these metal oxides are ionic. Ionic formation for transition metals is complicated by the fact that. Are Transition Metals Ionic.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica Are Transition Metals Ionic Therefore, these metal oxides are ionic. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. These include variable oxidation state (oxidation number), complex ion formation,. Are Transition Metals Ionic.
From slideplayer.com
Ionic Compounds Involving Transition Metals ppt download Are Transition Metals Ionic The field of complexes is. Therefore, these metal oxides are ionic. As a result, transition metals are able lose inner shell electrons, in addition to their valence electrons. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Transition metals in low oxidation states. Are Transition Metals Ionic.
From www.slideserve.com
PPT Polyatomic ions & Naming ionic Compounds PowerPoint Presentation Are Transition Metals Ionic As a result, transition metals are able lose inner shell electrons, in addition to their valence electrons. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. Transition metals in low oxidation states have lower electronegativity values than oxygen; The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds.. Are Transition Metals Ionic.
From www.youtube.com
Formulas and Names for Binary Ionic Compounds with Transition Metal Are Transition Metals Ionic Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Although the next higher \(s\) orbitals are. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. The formation of complexes causes the d orbitals to split into two. Are Transition Metals Ionic.
From www.youtube.com
Chemical Names and Formulas Part 2Transition Metals in Ionic Are Transition Metals Ionic Transition metals in low oxidation states have lower electronegativity values than oxygen; Therefore, these metal oxides are ionic. Embedded video, no tabs, this description appears on section page: Transition metals in low oxidation states have lower electronegativity values than oxygen; You will find some of this covered quite briefly on this page with. These include variable oxidation state (oxidation number),. Are Transition Metals Ionic.
From slideplayer.com
Day 8 Ionic compounds with transition metals ppt download Are Transition Metals Ionic Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. You will find some of this covered quite briefly on this page with. Therefore, these metal oxides are ionic. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific. Are Transition Metals Ionic.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download Are Transition Metals Ionic As a result, transition metals are able lose inner shell electrons, in addition to their valence electrons. Embedded video, no tabs, this description appears on section page: Therefore, these metal oxides are ionic. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. Although the next higher \(s\) orbitals are. Transition metals in low oxidation. Are Transition Metals Ionic.
From www.youtube.com
Transition Metal Ionic Compound Names and Formulas YouTube Are Transition Metals Ionic Therefore, these metal oxides are ionic. You will find some of this covered quite briefly on this page with. Transition metals in low oxidation states have lower electronegativity values than oxygen; Therefore, these metal oxides are ionic. The field of complexes is. Transition metals in low oxidation states have lower electronegativity values than oxygen; As a result, transition metals are. Are Transition Metals Ionic.
From www.slideserve.com
PPT Ionic Compounds Containing Multivalent Ions PowerPoint Are Transition Metals Ionic You will find some of this covered quite briefly on this page with. Transition metals in low oxidation states have lower electronegativity values than oxygen; Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. Transition metals in low oxidation states have lower electronegativity values than oxygen;. Are Transition Metals Ionic.
From www.slideserve.com
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free Are Transition Metals Ionic Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Therefore, these metal oxides. Are Transition Metals Ionic.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry I Are Transition Metals Ionic Although the next higher \(s\) orbitals are. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Transition metals in low oxidation states have lower electronegativity. Are Transition Metals Ionic.
From www.youtube.com
Ionic Naming with Transition Metals YouTube Are Transition Metals Ionic The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Embedded video, no. Are Transition Metals Ionic.
From app.jove.com
Transition Metals Electron Configurations and Properties Concept Are Transition Metals Ionic Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the. Are Transition Metals Ionic.
From www.slideserve.com
PPT Ionic Compounds Containing Multivalent Ions PowerPoint Are Transition Metals Ionic The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Transition metals in low oxidation states have lower electronegativity values than oxygen; Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or. Are Transition Metals Ionic.
From slideplayer.com
Ionic Compounds Involving Transition Metals ppt download Are Transition Metals Ionic As a result, transition metals are able lose inner shell electrons, in addition to their valence electrons. Therefore, these metal oxides are ionic. Although the next higher \(s\) orbitals are. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3) 2+ are referred to as complex ions or complexes. The formation of complexes causes the d. Are Transition Metals Ionic.
From utedzz.blogspot.com
Printable Periodic Table Ionic Charges Periodic Table Timeline Are Transition Metals Ionic Although the next higher \(s\) orbitals are. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. Ionic formation for transition metals is complicated by the fact that these elements have unfilled inner \(d\) shells. Therefore, these metal oxides are ionic. Species such as ni (oh 2) 62+ and ni (oh 2) 5 (nh 3). Are Transition Metals Ionic.