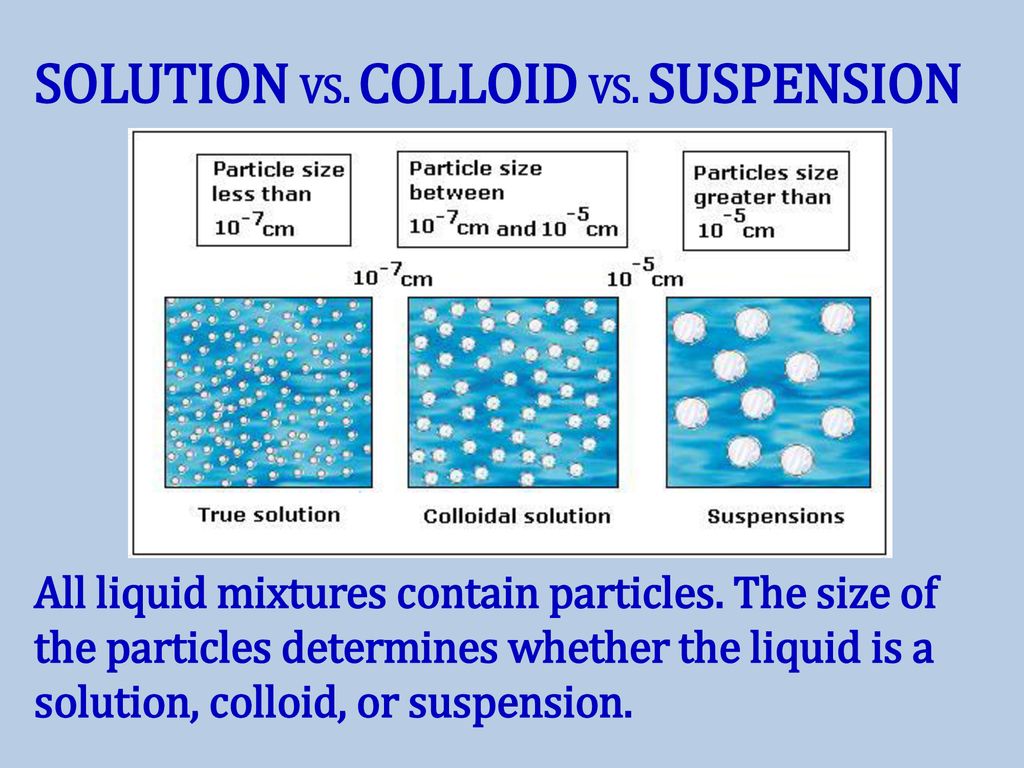
Suspension Colloid And Solution . Therefore, from the smallest particle to the largest, the correct order is: A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. So, the particles in a suspension typically settle out of their. Compare colloids, suspensions, solutions, and find their differences. In colloids, the particles that are. The particles in a suspension are larger than in a colloid. To distinguish between true solutions and solutions with aggregate particles. Solutions exhibit completely different behavior from suspensions. Difference between a colloid and a suspension. Understand particles in a solution, and explore solution, suspension, and colloid examples. A solution may be colored, but it is transparent, the molecules or ions are. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve.
from
Compare colloids, suspensions, solutions, and find their differences. The particles in a suspension are larger than in a colloid. Difference between a colloid and a suspension. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. Therefore, from the smallest particle to the largest, the correct order is: A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A solution may be colored, but it is transparent, the molecules or ions are. To distinguish between true solutions and solutions with aggregate particles. Understand particles in a solution, and explore solution, suspension, and colloid examples. So, the particles in a suspension typically settle out of their.
Suspension Colloid And Solution Understand particles in a solution, and explore solution, suspension, and colloid examples. To distinguish between true solutions and solutions with aggregate particles. Compare colloids, suspensions, solutions, and find their differences. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. The particles in a suspension are larger than in a colloid. Understand particles in a solution, and explore solution, suspension, and colloid examples. A solution may be colored, but it is transparent, the molecules or ions are. Solutions exhibit completely different behavior from suspensions. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. In colloids, the particles that are. So, the particles in a suspension typically settle out of their. Difference between a colloid and a suspension. Therefore, from the smallest particle to the largest, the correct order is:
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Suspension Colloid And Solution Therefore, from the smallest particle to the largest, the correct order is: A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. Compare colloids, suspensions, solutions, and find their differences. A solution may be colored, but it is transparent, the molecules or ions are. So, the particles in a suspension. Suspension Colloid And Solution.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Suspension Colloid And Solution Therefore, from the smallest particle to the largest, the correct order is: Solutions exhibit completely different behavior from suspensions. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. The particles in a suspension are larger than in a colloid. Compare colloids, suspensions, solutions, and find their differences. A colloid. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. Compare colloids, suspensions, solutions, and find their differences. Therefore, from the smallest particle to the largest, the correct order is: Difference between a colloid and a suspension. A solution may be colored, but it is transparent, the molecules or ions. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Difference between a colloid and a suspension. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. The particles in a suspension are larger than in a colloid. To distinguish between true solutions and solutions with aggregate particles. A colloid is a heterogeneous mixture in which the dispersed particles are. Suspension Colloid And Solution.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Colloid And Solution Compare colloids, suspensions, solutions, and find their differences. The particles in a suspension are larger than in a colloid. Solutions exhibit completely different behavior from suspensions. Understand particles in a solution, and explore solution, suspension, and colloid examples. Difference between a colloid and a suspension. In colloids, the particles that are. A solution may be colored, but it is transparent,. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A solution may be colored, but it is transparent, the molecules or ions are. So, the particles in a suspension typically settle out of their. Understand particles in a solution, and explore solution, suspension, and colloid examples. In colloids, the particles that are. Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Compare colloids, suspensions, solutions, and find their differences. Solutions exhibit completely different behavior from suspensions. To distinguish between true solutions and solutions with aggregate particles. In colloids, the particles that are. So, the particles in a suspension typically. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Difference between a colloid and a suspension. Solutions exhibit completely different behavior from suspensions. So, the particles in a suspension typically settle out of their. To distinguish between true solutions and solutions with aggregate particles. Therefore, from the smallest particle to the largest, the correct order is: Compare colloids, suspensions, solutions, and find their differences. A suspension is a heterogeneous. Suspension Colloid And Solution.
From
Suspension Colloid And Solution To distinguish between true solutions and solutions with aggregate particles. A solution may be colored, but it is transparent, the molecules or ions are. Difference between a colloid and a suspension. The particles in a suspension are larger than in a colloid. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do. Suspension Colloid And Solution.
From
Suspension Colloid And Solution To distinguish between true solutions and solutions with aggregate particles. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Difference between a colloid and a suspension. Compare. Suspension Colloid And Solution.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Colloid And Solution Compare colloids, suspensions, solutions, and find their differences. In colloids, the particles that are. A solution may be colored, but it is transparent, the molecules or ions are. To distinguish between true solutions and solutions with aggregate particles. Understand particles in a solution, and explore solution, suspension, and colloid examples. Difference between a colloid and a suspension. The particles in. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Therefore, from the smallest particle to the largest, the correct order is: So, the particles in a suspension typically settle out of their. Compare colloids, suspensions, solutions, and find their differences. To distinguish between true solutions and solutions with aggregate particles. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not. Suspension Colloid And Solution.
From ar.inspiredpencil.com
Colloid Suspension Solution Suspension Colloid And Solution To distinguish between true solutions and solutions with aggregate particles. So, the particles in a suspension typically settle out of their. Solutions exhibit completely different behavior from suspensions. Understand particles in a solution, and explore solution, suspension, and colloid examples. In colloids, the particles that are. Therefore, from the smallest particle to the largest, the correct order is: Compare colloids,. Suspension Colloid And Solution.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Suspension Colloid And Solution Difference between a colloid and a suspension. Therefore, from the smallest particle to the largest, the correct order is: So, the particles in a suspension typically settle out of their. Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Understand. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A solution may be colored, but it is transparent, the molecules or ions are. Solutions exhibit completely different behavior from suspensions. Compare colloids, suspensions, solutions, and find their differences. A suspension is a heterogeneous mixture in which particles. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Understand particles in a solution, and explore solution, suspension, and colloid examples. To distinguish between true solutions and solutions with aggregate particles. Difference between a colloid and a suspension. Therefore, from the smallest particle to the largest, the correct order is: Compare colloids, suspensions, solutions, and find their differences. A solution may be colored, but it is transparent, the molecules. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Compare colloids, suspensions, solutions, and find their differences. Understand particles in a solution, and explore solution, suspension, and colloid examples. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. A solution may be colored, but it is transparent, the molecules or ions are. Therefore, from the smallest particle to. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. Solutions exhibit completely different behavior from suspensions. The particles in a suspension are larger than in a colloid. So, the particles in a suspension typically settle out of their. Understand particles in a solution, and explore solution, suspension, and colloid. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. In colloids, the particles that are. Difference between a colloid and a suspension. To distinguish between true solutions and solutions with aggregate particles. Compare colloids, suspensions, solutions, and find their differences. A solution may be colored, but it is transparent,. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. In colloids, the particles that are. Understand particles in a solution, and explore solution, suspension, and colloid examples. So, the particles in a suspension typically settle out of their. Compare colloids,. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A solution may be colored, but it is transparent, the molecules or ions are. Compare colloids, suspensions, solutions, and find their differences. Therefore, from the smallest particle to the largest, the correct order is: The particles in a suspension are larger than in a colloid. Difference between a colloid and a suspension. In colloids, the particles that are. To distinguish. Suspension Colloid And Solution.
From
Suspension Colloid And Solution The particles in a suspension are larger than in a colloid. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. Compare colloids, suspensions, solutions, and find their differences. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Therefore, from the smallest particle to the largest, the correct order is: Understand particles in a solution, and explore solution, suspension, and colloid examples. The particles in a suspension are larger. Suspension Colloid And Solution.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Colloid And Solution Therefore, from the smallest particle to the largest, the correct order is: Understand particles in a solution, and explore solution, suspension, and colloid examples. So, the particles in a suspension typically settle out of their. Solutions exhibit completely different behavior from suspensions. Difference between a colloid and a suspension. Compare colloids, suspensions, solutions, and find their differences. The particles in. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Understand particles in a solution, and explore solution, suspension, and colloid examples. Compare colloids, suspensions, solutions, and find their differences. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. So, the particles in a suspension typically settle out of their. Therefore, from the smallest particle to the largest, the. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Solutions exhibit completely different behavior from suspensions. A solution may be colored, but it is transparent, the molecules or ions are. Understand particles in a solution, and explore solution, suspension, and colloid examples. Compare colloids, suspensions, solutions, and find their differences. Difference between a colloid and a suspension. Therefore, from the smallest particle to the largest, the correct order is:. Suspension Colloid And Solution.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspension Colloid And Solution Solutions exhibit completely different behavior from suspensions. Difference between a colloid and a suspension. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. So, the particles in a suspension typically settle out of their. To distinguish between true solutions and solutions with aggregate particles. The particles in a suspension. Suspension Colloid And Solution.
From brainly.in
what is the different between solution suspension and colloid Brainly.in Suspension Colloid And Solution Compare colloids, suspensions, solutions, and find their differences. Solutions exhibit completely different behavior from suspensions. So, the particles in a suspension typically settle out of their. The particles in a suspension are larger than in a colloid. A solution may be colored, but it is transparent, the molecules or ions are. To distinguish between true solutions and solutions with aggregate. Suspension Colloid And Solution.
From
Suspension Colloid And Solution So, the particles in a suspension typically settle out of their. Therefore, from the smallest particle to the largest, the correct order is: The particles in a suspension are larger than in a colloid. In colloids, the particles that are. A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve.. Suspension Colloid And Solution.
From jasiahgrofreeman.blogspot.com
Is Glass Cleaner a Solution Colloid or Suspension Suspension Colloid And Solution A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid) but do not dissolve. To distinguish between true solutions and solutions with aggregate particles. So, the particles in a suspension typically settle out of their. Difference between a colloid and a suspension. Compare colloids, suspensions, solutions, and find their differences. Therefore, from the smallest. Suspension Colloid And Solution.
From
Suspension Colloid And Solution Therefore, from the smallest particle to the largest, the correct order is: Solutions exhibit completely different behavior from suspensions. A solution may be colored, but it is transparent, the molecules or ions are. Understand particles in a solution, and explore solution, suspension, and colloid examples. To distinguish between true solutions and solutions with aggregate particles. In colloids, the particles that. Suspension Colloid And Solution.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Colloid And Solution The particles in a suspension are larger than in a colloid. In colloids, the particles that are. To distinguish between true solutions and solutions with aggregate particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Compare colloids, suspensions, solutions, and find their differences. A solution may. Suspension Colloid And Solution.
From sciencenotes.org
What Is a Colloid? Definition and Examples Suspension Colloid And Solution To distinguish between true solutions and solutions with aggregate particles. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Therefore, from the smallest particle to the largest, the correct order is: Compare colloids, suspensions, solutions,. Suspension Colloid And Solution.
From
Suspension Colloid And Solution A solution may be colored, but it is transparent, the molecules or ions are. In colloids, the particles that are. Solutions exhibit completely different behavior from suspensions. So, the particles in a suspension typically settle out of their. Therefore, from the smallest particle to the largest, the correct order is: The particles in a suspension are larger than in a. Suspension Colloid And Solution.
From pt.slideshare.net
Solutions, suspensions, and colloids Suspension Colloid And Solution So, the particles in a suspension typically settle out of their. A solution may be colored, but it is transparent, the molecules or ions are. Solutions exhibit completely different behavior from suspensions. Therefore, from the smallest particle to the largest, the correct order is: A suspension is a heterogeneous mixture in which particles disperse within a liquid or gas (fluid). Suspension Colloid And Solution.