What Is Medical Device Class A . In this guide, i will provide you. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. What i am about to share with you is a guide to medical device regulatory classification. In japan, medical devices are classified into four classes based on the risk level; Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Class i (extremely low risk), class ii (low risk), class iii (medium.
from emmainternational.com
Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. In japan, medical devices are classified into four classes based on the risk level; Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. What i am about to share with you is a guide to medical device regulatory classification. In this guide, i will provide you. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Class i (extremely low risk), class ii (low risk), class iii (medium. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,.
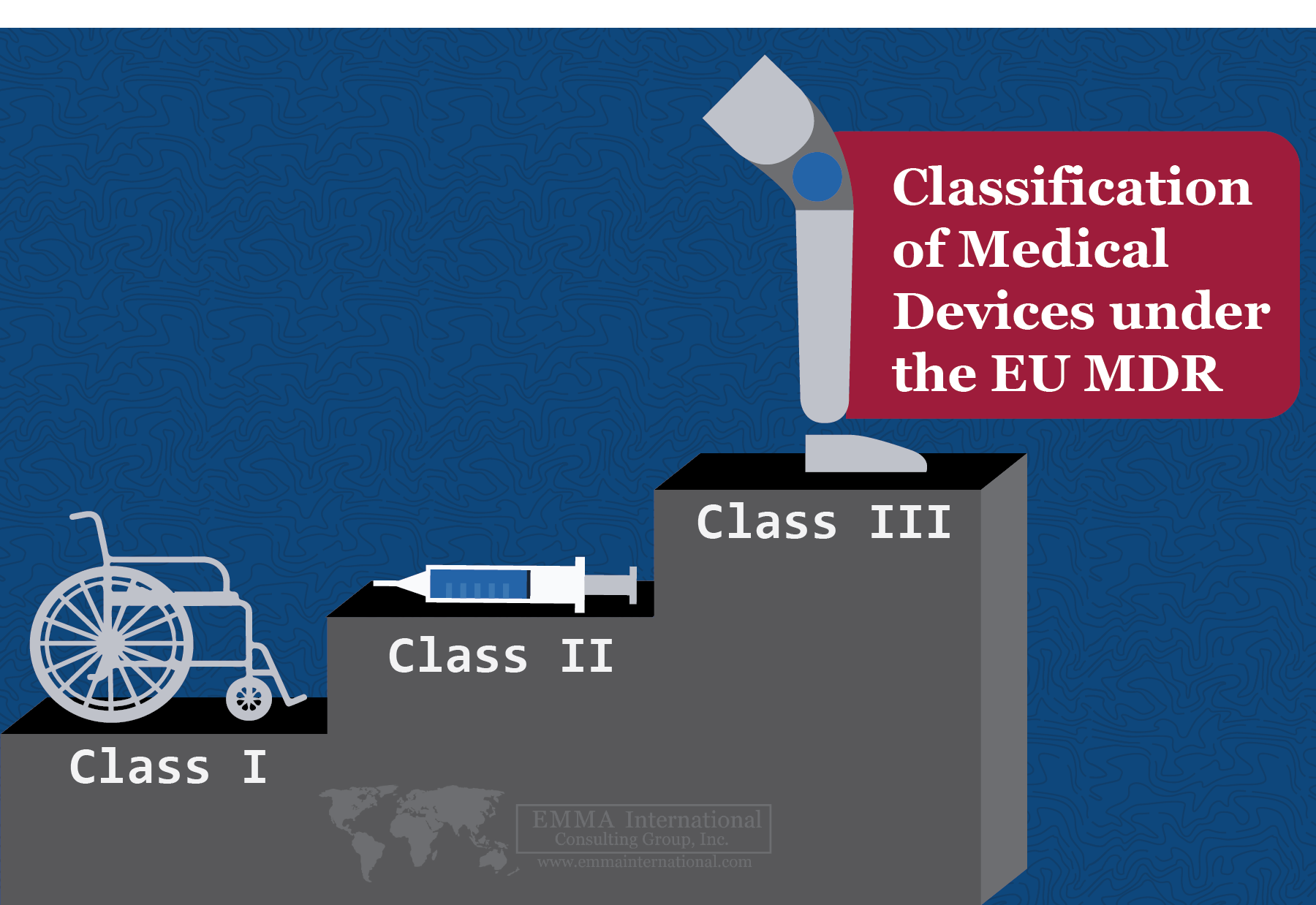
Classifying Medical Devices under EU MDR
What Is Medical Device Class A In japan, medical devices are classified into four classes based on the risk level; The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. In japan, medical devices are classified into four classes based on the risk level; In this guide, i will provide you. Class i (extremely low risk), class ii (low risk), class iii (medium. What i am about to share with you is a guide to medical device regulatory classification. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic.
From vem-medical.com
Medical Device Manufacturing What Is Medical Device Class A In japan, medical devices are classified into four classes based on the risk level; What i am about to share with you is a guide to medical device regulatory classification. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Any medical device approved by the fda center for devices. What Is Medical Device Class A.
From spyro-soft.com
A guide to FDA medical device regulations Spyrosoft What Is Medical Device Class A Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. In this guide, i will provide you. What i am about to share with you is a guide to medical device regulatory classification. Medical devices are assigned to one of three. What Is Medical Device Class A.
From www.rimsys.io
FDA listed, cleared, approved, granted what do these mean, and what’s What Is Medical Device Class A Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. What i am about to share with you is a guide to medical device regulatory classification. Class i (extremely low risk), class ii (low risk), class iii (medium. Any medical device approved by the fda center for devices and. What Is Medical Device Class A.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX What Is Medical Device Class A Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Class i (extremely low risk), class ii (low risk), class iii (medium. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Medical devices are assigned to one of. What Is Medical Device Class A.
From www.proximacro.com
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA What Is Medical Device Class A In japan, medical devices are classified into four classes based on the risk level; In this guide, i will provide you. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to. What Is Medical Device Class A.
From www.greenlight.guru
Ultimate Guide to Device Class Requirements under EU MDR What Is Medical Device Class A In japan, medical devices are classified into four classes based on the risk level; Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. In this guide, i will provide you. Determine if your product meets the definition of a medical. What Is Medical Device Class A.
From laegemiddelstyrelsen.dk
Medical devices What Is Medical Device Class A The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. In japan, medical devices are classified into four classes based on the risk level; Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Class i (extremely low risk),. What Is Medical Device Class A.
From cortex-design.com
Cortex Design • What's My FDA Medical Device Classification? What Is Medical Device Class A Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Any medical device approved by the fda center for devices and radiological health is classified as either. What Is Medical Device Class A.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group What Is Medical Device Class A What i am about to share with you is a guide to medical device regulatory classification. In this guide, i will provide you. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. In japan, medical devices are classified into four classes based on the risk level; Any. What Is Medical Device Class A.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations What Is Medical Device Class A Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. In japan, medical devices are classified into four classes based on the risk level; Class i (extremely low risk), class ii (low risk), class iii (medium. In this guide, i will provide you. What i am about to. What Is Medical Device Class A.
From omcmedical.com
Classification of Medical Devices Based on UK MDR 2002 What Is Medical Device Class A Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Class i (extremely low risk), class ii (low risk), class iii (medium. What i am about to share. What Is Medical Device Class A.
From operonstrategist.com
FDA Medical Device Classification Guide (Determine Your Device Class What Is Medical Device Class A Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. In japan, medical devices are classified into four classes based on. What Is Medical Device Class A.
From www.evidera.com
White Paper The Growing Need for RealWorld Evidence in Medical What Is Medical Device Class A In this guide, i will provide you. Class i (extremely low risk), class ii (low risk), class iii (medium. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. In japan, medical devices are classified into four classes based on the risk level; The product code assigned to a. What Is Medical Device Class A.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX What Is Medical Device Class A Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. In japan, medical devices are classified into four classes based on the risk level; The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. In this guide, i will. What Is Medical Device Class A.
From emmainternational.com
Classifying Medical Devices under EU MDR What Is Medical Device Class A Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. Class i (extremely low risk), class ii (low risk), class iii (medium. In japan, medical devices are classified into four classes based on the risk level; Determine if your product meets. What Is Medical Device Class A.
From medicaldevicehq.com
Different classifications rules for medical device software An What Is Medical Device Class A Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Class i (extremely low risk), class ii (low risk), class iii (medium. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Medical devices are assigned to one of. What Is Medical Device Class A.
From www.regdesk.co
FDA Exemption for Class II Medical Devices RegDesk What Is Medical Device Class A Class i (extremely low risk), class ii (low risk), class iii (medium. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new. What Is Medical Device Class A.
From www.arenasolutions.com
Medical Device Definition Arena What Is Medical Device Class A The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. What i am about to share with you is a guide. What Is Medical Device Class A.
From blog.chino.io
What MDR class is my eHealth app? What Is Medical Device Class A Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. What i am about to share with you is a guide to medical device regulatory classification. In japan, medical. What Is Medical Device Class A.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market What Is Medical Device Class A Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. In japan, medical devices are classified into four classes based on the risk level; Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. In this guide,. What Is Medical Device Class A.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation What Is Medical Device Class A Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. What i am about to share with you is a guide to medical device regulatory classification. Determine if your product meets the definition of a medical device per section 201 (h). What Is Medical Device Class A.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero What Is Medical Device Class A The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. In this guide, i will provide you. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. Class i (extremely low risk), class ii (low risk), class iii. What Is Medical Device Class A.
From www.greenlight.guru
What is an FDA Class 1 Medical Device? [+Examples] What Is Medical Device Class A In japan, medical devices are classified into four classes based on the risk level; Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. Medical devices are assigned to one of three regulatory classes based on the level of control necessary. What Is Medical Device Class A.
From www.regdesk.co
What Is A Class 1 Medical Device RegDesk What Is Medical Device Class A Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. In japan, medical devices are classified into four classes based on the risk level; Determine if your product meets the definition of a medical device per section 201 (h) of the. What Is Medical Device Class A.
From kantify.ai
Kantify Improving Human Health through Artificial Intelligence What Is Medical Device Class A What i am about to share with you is a guide to medical device regulatory classification. Class i (extremely low risk), class ii (low risk), class iii (medium. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. The product code assigned to a device is based upon the. What Is Medical Device Class A.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is Medical Device Class A In this guide, i will provide you. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Determine if your product. What Is Medical Device Class A.
From operonstrategist.com
Classifying a Class III Medical Device Process) Operon What Is Medical Device Class A In japan, medical devices are classified into four classes based on the risk level; Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. In this guide, i. What Is Medical Device Class A.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market What Is Medical Device Class A The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Class i (extremely low risk), class ii (low risk), class iii (medium. In this guide, i will provide you.. What Is Medical Device Class A.
From angelanjohnson.com
Medical Devices Angela N Johnson What Is Medical Device Class A In this guide, i will provide you. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Class i (extremely low risk), class ii (low risk), class iii (medium. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or. What Is Medical Device Class A.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class What Is Medical Device Class A Class i (extremely low risk), class ii (low risk), class iii (medium. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug. What Is Medical Device Class A.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft What Is Medical Device Class A Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. What i am about to share with you is a guide to medical device regulatory classification. In japan, medical devices are classified into four classes based on the risk level; Class i (extremely low risk), class ii (low risk),. What Is Medical Device Class A.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment What Is Medical Device Class A In this guide, i will provide you. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. In japan, medical devices are classified into four classes based on the risk level; Determine if your product meets the definition of a medical device per section 201 (h) of the. What Is Medical Device Class A.
From cortex-design.com
Cortex Design • What's My FDA Medical Device Classification? What Is Medical Device Class A In this guide, i will provide you. Class i (extremely low risk), class ii (low risk), class iii (medium. What i am about to share with you is a guide to medical device regulatory classification. The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Any medical device approved by. What Is Medical Device Class A.
From lifechanginginnovation.org
What is a Medical Device? Life Changing Innovation What Is Medical Device Class A The product code assigned to a device is based upon the medical device product classification designated under 21 cfr parts. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness,. In japan, medical devices are classified into four classes based on. What Is Medical Device Class A.
From www.vrogue.co
What Is The Fda Definition Of A Medical Device Class vrogue.co What Is Medical Device Class A In this guide, i will provide you. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. In japan, medical devices are classified into four classes based on the risk level; What i am about to share with you is a guide to medical device regulatory classification. The. What Is Medical Device Class A.