What Is The Ph Of Common Salt Solution . a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. — contents show. the ph depends on the relative strengths of the acid and base. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? calculating ph of a salt solution. — calculating ph of salt solutions. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. When certain soluble salts are dissolved in water the resulting solution is. In science we often use the term aqueous solution. It is often helpful to be able to predict the effect a salt solution will have.
from www.chegg.com
a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — calculating ph of salt solutions. the ph depends on the relative strengths of the acid and base. calculating ph of a salt solution. In science we often use the term aqueous solution. It is often helpful to be able to predict the effect a salt solution will have. When certain soluble salts are dissolved in water the resulting solution is. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? — contents show. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak.
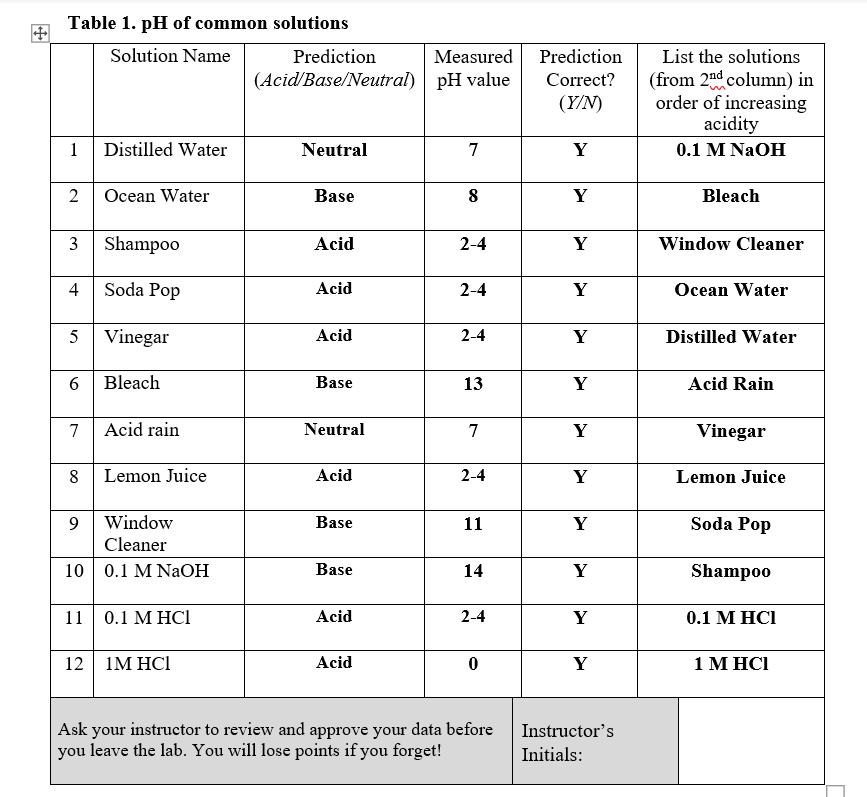
Solved Table 1. pH of common solutions Solution Name
What Is The Ph Of Common Salt Solution calculating ph of a salt solution. It is often helpful to be able to predict the effect a salt solution will have. — calculating ph of salt solutions. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. — contents show. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. the ph depends on the relative strengths of the acid and base. calculating ph of a salt solution. When certain soluble salts are dissolved in water the resulting solution is. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? In science we often use the term aqueous solution.
From www.slideserve.com
PPT pH of Salts Solutions (Hydrolysis) PowerPoint Presentation, free What Is The Ph Of Common Salt Solution the ph depends on the relative strengths of the acid and base. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? — calculating ph of salt solutions. It is often helpful to be able to predict the effect a salt solution will have.. What Is The Ph Of Common Salt Solution.
From www.youtube.com
pH of Salts (लवणों का pH) pHofsalts salts YouTube What Is The Ph Of Common Salt Solution — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? When certain soluble salts are dissolved in water the resulting solution is. the ph depends on the relative strengths of the acid and base. It is often helpful to be able to predict the effect. What Is The Ph Of Common Salt Solution.
From printablelibsleety.z13.web.core.windows.net
How To Find Ph Of Salt What Is The Ph Of Common Salt Solution — contents show. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. the ph depends on the relative strengths of the acid and base. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. A general idea behind the neutrality of sodium. What Is The Ph Of Common Salt Solution.
From www.youtube.com
physiological salt solution types, composition and uses YouTube What Is The Ph Of Common Salt Solution In science we often use the term aqueous solution. It is often helpful to be able to predict the effect a salt solution will have. When certain soluble salts are dissolved in water the resulting solution is. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic. What Is The Ph Of Common Salt Solution.
From www.youtube.com
pH of Salt Solutions YouTube What Is The Ph Of Common Salt Solution calculating ph of a salt solution. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. It is often helpful to be able to predict the effect a salt solution will have. — contents show. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. the ph depends on. What Is The Ph Of Common Salt Solution.
From www.youtube.com
Calculating the pH of a Salt Solution YouTube What Is The Ph Of Common Salt Solution A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. It is often helpful to be able to predict the effect a salt solution will have. the ph depends on the relative strengths of the acid and base. calculating ph of a salt solution. — why does a. What Is The Ph Of Common Salt Solution.
From www.youtube.com
CHAPTER 16, Slides 84 to 101, pH of Salt Solutions YouTube What Is The Ph Of Common Salt Solution In science we often use the term aqueous solution. calculating ph of a salt solution. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. When. What Is The Ph Of Common Salt Solution.
From learningschoolzazobezx.z22.web.core.windows.net
Why Do Salt Solutions Have A Ph What Is The Ph Of Common Salt Solution In science we often use the term aqueous solution. calculating ph of a salt solution. When certain soluble salts are dissolved in water the resulting solution is. It is often helpful to be able to predict the effect a salt solution will have. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. — why does a salt containing. What Is The Ph Of Common Salt Solution.
From dxodumcjf.blob.core.windows.net
What Is The Ph Of Kosher Salt at Linda Hankinson blog What Is The Ph Of Common Salt Solution — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? — contents show. It is often helpful to be able to predict the effect a salt solution will have. a salt that is derived from the reaction of a strong acid with a strong. What Is The Ph Of Common Salt Solution.
From study.com
Acidic, Basic & Neutral Solutions Overview, pH Scale & Uses Lesson What Is The Ph Of Common Salt Solution a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. calculating ph of a salt solution. the ph depends on the relative strengths of the acid and base. —. What Is The Ph Of Common Salt Solution.
From byjus.com
31. What is the pH of salt of strong acid and weak base? What Is The Ph Of Common Salt Solution calculating ph of a salt solution. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. — calculating ph of salt solutions. It is often helpful to be able to predict the effect a salt solution will have. —. What Is The Ph Of Common Salt Solution.
From exofbishk.blob.core.windows.net
What Is The Ph Of Salt Water Solution at Travis Farley blog What Is The Ph Of Common Salt Solution It is often helpful to be able to predict the effect a salt solution will have. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. In science we often use the term aqueous solution. calculating ph of a salt solution. the ph depends on the relative strengths of. What Is The Ph Of Common Salt Solution.
From www.slideserve.com
PPT pH of Salts PowerPoint Presentation, free download ID1994692 What Is The Ph Of Common Salt Solution A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. calculating ph of a salt solution. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. It is often helpful to be able to. What Is The Ph Of Common Salt Solution.
From www.slideserve.com
PPT pH of Salts PowerPoint Presentation, free download ID2866899 What Is The Ph Of Common Salt Solution the ph depends on the relative strengths of the acid and base. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. It is often helpful to be able to predict the effect a salt solution will have. — contents show. — why does a salt containing a. What Is The Ph Of Common Salt Solution.
From www.youtube.com
16.9 pH of Salt Solution Example Problem YouTube What Is The Ph Of Common Salt Solution calculating ph of a salt solution. the ph depends on the relative strengths of the acid and base. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. — contents show. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. a solution of common salt (sodium chloride). What Is The Ph Of Common Salt Solution.
From manuallistsmugglers.z21.web.core.windows.net
Use A Diagram To Explain Ph Scale What Is The Ph Of Common Salt Solution — contents show. When certain soluble salts are dissolved in water the resulting solution is. — calculating ph of salt solutions. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. In science we often use the term aqueous solution.. What Is The Ph Of Common Salt Solution.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt What Is The Ph Of Common Salt Solution — calculating ph of salt solutions. It is often helpful to be able to predict the effect a salt solution will have. In science we often use the term aqueous solution. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. — contents show. a solution of common salt (sodium chloride) is also neutral, so it has a. What Is The Ph Of Common Salt Solution.
From chemistryguru.com.sg
Calculate pH of Salt Solution What Is The Ph Of Common Salt Solution the ph depends on the relative strengths of the acid and base. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. a salt that is derived from the reaction of a strong acid with a strong base forms a. What Is The Ph Of Common Salt Solution.
From learningschoolzazobezx.z22.web.core.windows.net
Why Do Salt Solutions Have A Ph What Is The Ph Of Common Salt Solution calculating ph of a salt solution. the ph depends on the relative strengths of the acid and base. In science we often use the term aqueous solution. — contents show. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? — calculating. What Is The Ph Of Common Salt Solution.
From www.chegg.com
Solved Table 1. pH of common solutions Solution Name What Is The Ph Of Common Salt Solution the ph depends on the relative strengths of the acid and base. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. — why does a salt containing a cation. What Is The Ph Of Common Salt Solution.
From www.mometrix.com
pH Overview (Chemistry Review Video) What Is The Ph Of Common Salt Solution a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — calculating ph of salt solutions. When certain soluble salts are dissolved in water the resulting solution is. a solution of common salt (sodium chloride) is also neutral, so it has a. What Is The Ph Of Common Salt Solution.
From www.bbc.co.uk
What is the pH scale and what does it measure? BBC Bitesize What Is The Ph Of Common Salt Solution a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. In science we often use the term aqueous solution. the ph depends on the relative strengths of the acid and base. a salt that is derived from the reaction of a strong acid with a strong base forms a. What Is The Ph Of Common Salt Solution.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision What Is The Ph Of Common Salt Solution a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? When certain soluble salts are dissolved in water the resulting. What Is The Ph Of Common Salt Solution.
From www.slideserve.com
PPT Chapter 2 Chemical Principles PowerPoint Presentation, free What Is The Ph Of Common Salt Solution calculating ph of a salt solution. In science we often use the term aqueous solution. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. It is often helpful to be able to predict the effect a salt solution will have. — why does a salt containing a cation. What Is The Ph Of Common Salt Solution.
From www.youtube.com
pH of a Salt Solution YouTube What Is The Ph Of Common Salt Solution It is often helpful to be able to predict the effect a salt solution will have. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — contents show. In science we often use the term aqueous solution. — why does a. What Is The Ph Of Common Salt Solution.
From studylib.net
pH of Salt Solutions and Buffers What Is The Ph Of Common Salt Solution Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. calculating ph of a salt solution. the ph depends on the relative strengths of the acid and base. — contents show. When certain soluble salts are dissolved in water. What Is The Ph Of Common Salt Solution.
From www.youtube.com
Explaining the pH of Salt Solutions YouTube What Is The Ph Of Common Salt Solution a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. the ph depends on the relative strengths of the acid and base. When certain. What Is The Ph Of Common Salt Solution.
From www.slideserve.com
PPT pH of Salts Solutions (Hydrolysis) PowerPoint Presentation, free What Is The Ph Of Common Salt Solution a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. — calculating ph of salt solutions. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. . What Is The Ph Of Common Salt Solution.
From www.oist.jp
pH Values of Common Substances Okinawa Institute of Science and What Is The Ph Of Common Salt Solution — calculating ph of salt solutions. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — contents show. — why does. What Is The Ph Of Common Salt Solution.
From www.youtube.com
How to Calculate the pH of a Salt Solution Practice Problems, Shortcut What Is The Ph Of Common Salt Solution When certain soluble salts are dissolved in water the resulting solution is. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? a solution of common. What Is The Ph Of Common Salt Solution.
From general.chemistrysteps.com
Acidity of a Salt Solution Chemistry Steps What Is The Ph Of Common Salt Solution In science we often use the term aqueous solution. — contents show. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a.. What Is The Ph Of Common Salt Solution.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2008 PowerPoint Presentation, free What Is The Ph Of Common Salt Solution Nh₄cn, formed from hcn (weak acid) and nh₃ (weak. — calculating ph of salt solutions. A general idea behind the neutrality of sodium chloride is that the acidic, basic or neutral nature of a. In science we often use the term aqueous solution. a salt that is derived from the reaction of a strong acid with a strong. What Is The Ph Of Common Salt Solution.
From www.researchgate.net
pH values of chemical compounds Download Scientific Diagram What Is The Ph Of Common Salt Solution — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? When certain soluble salts are dissolved in water the resulting solution is. — contents show. a solution of common salt (sodium chloride) is also neutral, so it has a ph of 7 too. In. What Is The Ph Of Common Salt Solution.
From ar.inspiredpencil.com
Ph Scale Acids And Bases What Is The Ph Of Common Salt Solution It is often helpful to be able to predict the effect a salt solution will have. — calculating ph of salt solutions. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. When certain soluble salts are dissolved in water the resulting solution. What Is The Ph Of Common Salt Solution.
From www.youtube.com
Calculating pH of Salt Solutions YouTube What Is The Ph Of Common Salt Solution — why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? It is often helpful to be able to predict the effect a salt solution will have. the ph depends on the relative strengths of the acid and base. Nh₄cn, formed from hcn (weak acid) and. What Is The Ph Of Common Salt Solution.