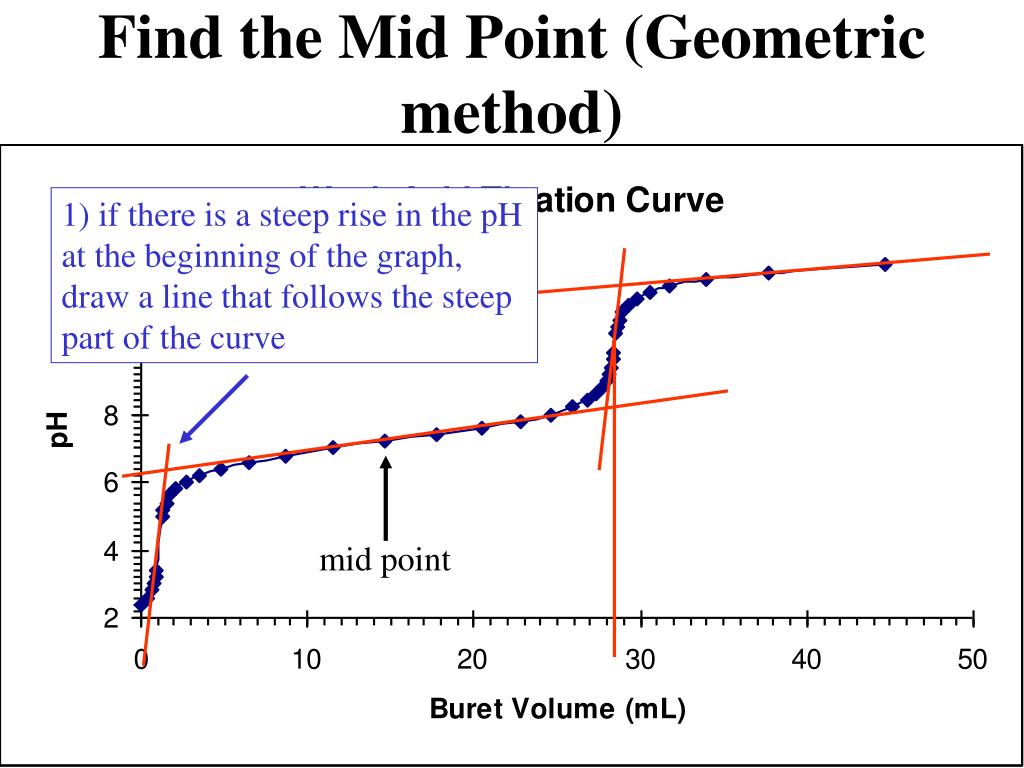
Titration Midpoint Meaning . The midpoint of the inflection is called the equivalence point. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. There are two basic types of acid base titrations, indicator and potentiometric. From the curves, you can: In an indicator based titration you add another chemical that changes. One point in the titration of a weak acid or a weak base is particularly important: A titration is a chemical analysis in which a researcher determines the concentration of a chemical.
from www.slideserve.com
One point in the titration of a weak acid or a weak base is particularly important: The midpoint of the inflection is called the equivalence point. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. In an indicator based titration you add another chemical that changes. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. From the curves, you can:
PPT How to Interpret Titration Curves PowerPoint Presentation, free
Titration Midpoint Meaning Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. From the curves, you can: Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. One point in the titration of a weak acid or a weak base is particularly important: In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. The midpoint of the inflection is called the equivalence point. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Midpoint Meaning One point in the titration of a weak acid or a weak base is particularly important: A titration is a chemical analysis in which a researcher determines the concentration of a chemical. The midpoint of the inflection is called the equivalence point. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base.. Titration Midpoint Meaning.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts Titration Midpoint Meaning A titration is a chemical analysis in which a researcher determines the concentration of a chemical. One point in the titration of a weak acid or a weak base is particularly important: There are two basic types of acid base titrations, indicator and potentiometric. From the curves, you can: Titration is a technique used in analytical chemistry to determine the. Titration Midpoint Meaning.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Midpoint Meaning The midpoint of the inflection is called the equivalence point. One point in the titration of a weak acid or a weak base is particularly important: From the curves, you can: Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. There are two basic types of acid base titrations, indicator and. Titration Midpoint Meaning.
From www.maieutapedia.org
Stoichiometry and Titration Maieuta Pedia Titration Midpoint Meaning A titration is a chemical analysis in which a researcher determines the concentration of a chemical. From the curves, you can: One point in the titration of a weak acid or a weak base is particularly important: In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric.. Titration Midpoint Meaning.
From chem4three.blogspot.co.uk
CHEMISTRY 11 TITRATIONS Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. One point in the titration of a weak acid or a weak base is particularly important: There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the. Titration Midpoint Meaning.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Midpoint Meaning Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the slow addition of one solution where the concentration is known to. Titration Midpoint Meaning.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Titration Midpoint Meaning One point in the titration of a weak acid or a weak base is particularly important: The midpoint of the inflection is called the equivalence point. There are two basic types of acid base titrations, indicator and potentiometric. From the curves, you can: Titration involves the slow addition of one solution where the concentration is known to a known volume. Titration Midpoint Meaning.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. The midpoint of the inflection is called the equivalence point. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. Titration involves the slow addition. Titration Midpoint Meaning.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Midpoint Meaning There are two basic types of acid base titrations, indicator and potentiometric. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. From the curves, you can: The midpoint of the inflection is called the equivalence point. A titration is a chemical analysis in which a researcher determines the concentration of a. Titration Midpoint Meaning.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titration Midpoint Meaning There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. In an indicator based titration you add another chemical that changes. One point in the titration. Titration Midpoint Meaning.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Midpoint Meaning There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. One point in the titration of a weak acid or a weak base is particularly important: The midpoint of. Titration Midpoint Meaning.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Midpoint Meaning From the curves, you can: Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes.. Titration Midpoint Meaning.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where. Titration Midpoint Meaning.
From www.writework.com
Titration of amino acids WriteWork Titration Midpoint Meaning There are two basic types of acid base titrations, indicator and potentiometric. The midpoint of the inflection is called the equivalence point. From the curves, you can: One point in the titration of a weak acid or a weak base is particularly important: A titration is a chemical analysis in which a researcher determines the concentration of a chemical. Titration. Titration Midpoint Meaning.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. One point in the titration of a weak acid or a weak base is particularly important: There are two basic types of acid base titrations, indicator and potentiometric. From the curves, you can:. Titration Midpoint Meaning.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Midpoint Meaning There are two basic types of acid base titrations, indicator and potentiometric. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. The midpoint of the inflection is called the equivalence point. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. Titration involves the slow. Titration Midpoint Meaning.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Midpoint Meaning There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. The midpoint of the inflection is called the equivalence point. One point in the titration of. Titration Midpoint Meaning.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Midpoint Meaning Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. From the curves, you can: In an indicator based titration you add another chemical that changes. The midpoint of the inflection is called the equivalence point. Titration is. Titration Midpoint Meaning.
From www.researchgate.net
Midpoint potential measurement of HLMWb5 with spectroelectrochemical Titration Midpoint Meaning There are two basic types of acid base titrations, indicator and potentiometric. From the curves, you can: One point in the titration of a weak acid or a weak base is particularly important: In an indicator based titration you add another chemical that changes. Titration involves the slow addition of one solution where the concentration is known to a known. Titration Midpoint Meaning.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. One point in the titration of a weak acid or a weak base is particularly important: Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. From the curves,. Titration Midpoint Meaning.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. From the curves, you can: One point in the titration of a weak acid or. Titration Midpoint Meaning.
From mavink.com
Titration Labeled Titration Midpoint Meaning A titration is a chemical analysis in which a researcher determines the concentration of a chemical. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. From the curves,. Titration Midpoint Meaning.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Midpoint Meaning A titration is a chemical analysis in which a researcher determines the concentration of a chemical. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. The midpoint of the inflection is called the equivalence point. From the curves, you can: There are two basic types of acid base titrations, indicator and. Titration Midpoint Meaning.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Midpoint Meaning One point in the titration of a weak acid or a weak base is particularly important: The midpoint of the inflection is called the equivalence point. In an indicator based titration you add another chemical that changes. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. There are two basic types. Titration Midpoint Meaning.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Midpoint Meaning The midpoint of the inflection is called the equivalence point. One point in the titration of a weak acid or a weak base is particularly important: Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. Titration involves the slow addition of one solution where the concentration is known to a known. Titration Midpoint Meaning.
From slidetodoc.com
How to Interpret Titration Curves find the equivalence Titration Midpoint Meaning Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. From the curves, you can: A titration is a chemical analysis in which a researcher determines the concentration of a chemical. The midpoint of the inflection is called. Titration Midpoint Meaning.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Midpoint Meaning Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. In an indicator based titration you add another chemical that changes. From the curves, you can: One point in the titration of a weak acid or a weak. Titration Midpoint Meaning.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Midpoint Meaning One point in the titration of a weak acid or a weak base is particularly important: From the curves, you can: In an indicator based titration you add another chemical that changes. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. There are two basic types of acid base titrations, indicator. Titration Midpoint Meaning.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Midpoint Meaning Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. In an indicator based titration you add another chemical that changes. One point in the titration of a weak acid or a weak base is particularly important: There. Titration Midpoint Meaning.
From www.youtube.com
Acid Base Titration Curves Simplified YouTube Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. The midpoint of the inflection is called the equivalence point. One point in the titration of a weak acid or a weak base is particularly important: From the curves, you can: A titration is a chemical analysis in which a researcher determines the concentration of a chemical. Titration is. Titration Midpoint Meaning.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Midpoint Meaning The midpoint of the inflection is called the equivalence point. A titration is a chemical analysis in which a researcher determines the concentration of a chemical. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. There are two basic types of acid base titrations, indicator and potentiometric. From the curves, you. Titration Midpoint Meaning.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Midpoint Meaning Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. In an indicator based titration you add another chemical that. Titration Midpoint Meaning.
From fernando-well-key.blogspot.com
How to Describe a Titration Curve Titration Midpoint Meaning Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. The midpoint of the inflection is called the equivalence point.. Titration Midpoint Meaning.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Midpoint Meaning In an indicator based titration you add another chemical that changes. Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. There are two basic types of acid base titrations, indicator and potentiometric. One point in the titration. Titration Midpoint Meaning.
From www.rdworldonline.com
What are titration instruments? Research & Development World Titration Midpoint Meaning Titration involves the slow addition of one solution where the concentration is known to a known volume of another solution where the concentration is unknown until the reaction reaches the desired level. One point in the titration of a weak acid or a weak base is particularly important: Titration is a technique used in analytical chemistry to determine the concentration. Titration Midpoint Meaning.