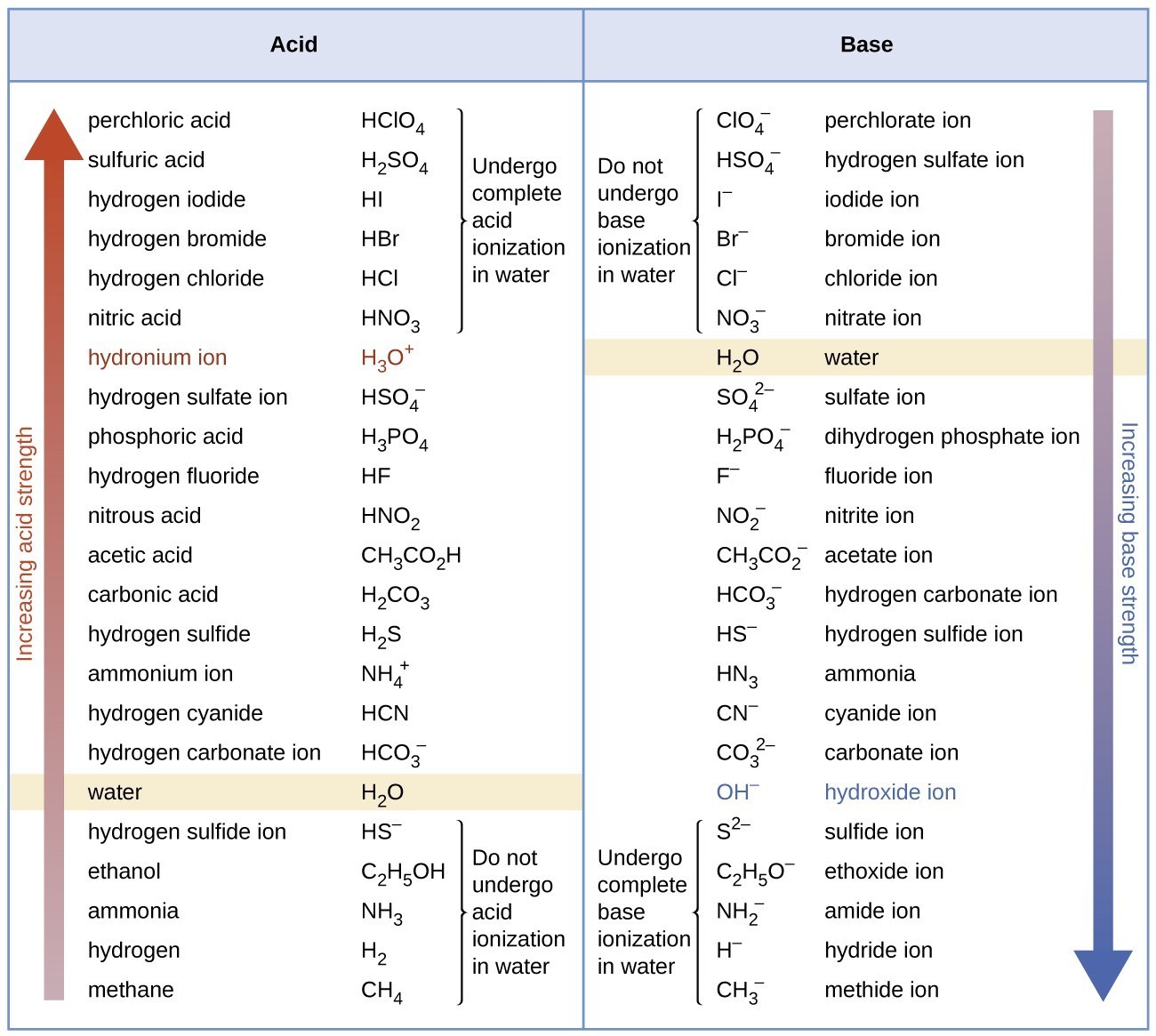
Acetic Acid Strong Or Weak . acetic acid ( ) is an example of a weak acid. When we add acetic acid to water, it explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. The strength of a weak acid is quantified by its acid dissociation constant, value. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. learn how to identify strong and weak acids and bases with definitions and examples. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph.
from courses.lumenlearning.com
explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. learn how to identify strong and weak acids and bases with definitions and examples. When we add acetic acid to water, it learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). acetic acid ( ) is an example of a weak acid. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. The strength of a weak acid is quantified by its acid dissociation constant, value.
Relative Strengths of Acids and Bases Chemistry
Acetic Acid Strong Or Weak learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. The strength of a weak acid is quantified by its acid dissociation constant, value. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. acetic acid ( ) is an example of a weak acid. When we add acetic acid to water, it Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. learn how to identify strong and weak acids and bases with definitions and examples. learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid).
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Acetic Acid Strong Or Weak thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. learn how to identify strong and weak acids and bases with definitions and examples. Find. Acetic Acid Strong Or Weak.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Acetic Acid Strong Or Weak thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). learn how to identify strong and weak acids and bases with definitions and examples. The strength of a weak acid is quantified by its acid dissociation constant, value. acetic acid ( ). Acetic Acid Strong Or Weak.
From strategiesforparents.com
Is Acetic Acid A Strong Acid or Weak Acid? Strategies for Parents Acetic Acid Strong Or Weak explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). Find out the 7 common strong acids and their properties, and how to. Acetic Acid Strong Or Weak.
From pediaa.com
Difference Between Acetic Acid and Citric Acid Definition, Properties Acetic Acid Strong Or Weak Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. learn how to identify strong and weak acids and bases with definitions and examples. learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. The strength of a weak acid is quantified by its acid dissociation constant, value. learn. Acetic Acid Strong Or Weak.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Acetic Acid Strong Or Weak learn how to identify strong and weak acids and bases with definitions and examples. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. acetic acid ( ) is an example of a weak acid. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as. Acetic Acid Strong Or Weak.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Acetic Acid Strong Or Weak acetic acid ( ) is an example of a weak acid. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. learn how to identify strong and weak acids and bases with. Acetic Acid Strong Or Weak.
From learningschoolzazobezx.z22.web.core.windows.net
How To Identify Bases And Acids Acetic Acid Strong Or Weak learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). explore the topics and concepts of chemistry with interactive flexbooks, simulations,. Acetic Acid Strong Or Weak.
From www.teachoo.com
Uses of Acids 10+ Examples (with Images) Teachoo Teachoo Questio Acetic Acid Strong Or Weak thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. The strength of a weak acid is quantified by its acid dissociation constant, value. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and. Acetic Acid Strong Or Weak.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid Acetic Acid Strong Or Weak learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. The strength. Acetic Acid Strong Or Weak.
From www.sciencephoto.com
Acetic acid molecule Stock Image F012/5730 Science Photo Library Acetic Acid Strong Or Weak acetic acid ( ) is an example of a weak acid. learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. The strength of a weak acid is quantified by its acid dissociation constant, value. Find out. Acetic Acid Strong Or Weak.
From www.coffeechemistry.com
Acetic Acid Acetic Acid Strong Or Weak learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. When we add acetic acid to water, it learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. explore the topics and concepts of chemistry with interactive flexbooks, simulations,. Acetic Acid Strong Or Weak.
From pediaa.com
Difference Between Formic Acid and Acetic Acid Definition, Properties Acetic Acid Strong Or Weak Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). When we add acetic acid to water, it . Acetic Acid Strong Or Weak.
From www.dreamstime.com
Acetic Acid, Structural Chemical Formula Stock Photo Image of Acetic Acid Strong Or Weak learn how to identify strong and weak acids and bases with definitions and examples. acetic acid ( ) is an example of a weak acid. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. The strength of a weak acid is quantified. Acetic Acid Strong Or Weak.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Acetic Acid Strong Or Weak When we add acetic acid to water, it learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. learn how to identify strong and weak acids and bases with definitions and examples. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in. Acetic Acid Strong Or Weak.
From www.thoughtco.com
List of Common Strong and Weak Acids Acetic Acid Strong Or Weak explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. learn how to identify strong and weak acids and bases with definitions and examples. learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. Find out the 7 common strong acids and their. Acetic Acid Strong Or Weak.
From www.pinterest.jp
Strong and weak acids collection set with educational diagram outline Acetic Acid Strong Or Weak explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. When we add acetic acid to water, it learn how to identify strong and weak acids and bases with definitions and examples. Find. Acetic Acid Strong Or Weak.
From www.youtube.com
Acetic Acid (CH3COOH) 3D Model with Lewis Structure YouTube Acetic Acid Strong Or Weak Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. When we add acetic acid to water, it The strength of a weak acid is quantified by its acid dissociation constant, value. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. acetic acid ( ) is an example of a weak acid. . Acetic Acid Strong Or Weak.
From www.vrogue.co
Why Hydrochloric Acid A Strong Acid And Acetic Acid A vrogue.co Acetic Acid Strong Or Weak When we add acetic acid to water, it learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. explore the topics and concepts of chemistry with interactive flexbooks, simulations,. Acetic Acid Strong Or Weak.
From acetic-acid.net
Why is Acetic Acid A Weak Acid? Understanding the Nature Acetic Acid Strong Or Weak learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry. Acetic Acid Strong Or Weak.
From www.thoughtco.com
Weak Acid Definition and Examples in Chemistry Acetic Acid Strong Or Weak acetic acid ( ) is an example of a weak acid. learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. learn the difference between strong acids and weak acids, how they. Acetic Acid Strong Or Weak.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry Acetic Acid Strong Or Weak thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. acetic acid ( ) is an example of a weak acid. learn how to identify strong and weak acids and bases with definitions and examples.. Acetic Acid Strong Or Weak.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6776086 Acetic Acid Strong Or Weak Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. The strength of a weak acid is quantified by its acid dissociation constant, value. When we add acetic acid to. Acetic Acid Strong Or Weak.
From passchemistry.com
Types of Intermolecular Forces Acetic Acid Strong Or Weak learn the difference between strong acids and weak acids, how they dissociate in water, and their applications in chemistry and industry. acetic acid ( ) is an example of a weak acid. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. thus, a weak acid increases the hydronium ion concentration in an. Acetic Acid Strong Or Weak.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Acetic Acid Strong Or Weak learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. learn how to identify strong and weak acids and bases with definitions and examples. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. acetic acid ( ) is an example of. Acetic Acid Strong Or Weak.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry Acetic Acid Strong Or Weak learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). explore the topics and concepts of chemistry with interactive flexbooks, simulations,. Acetic Acid Strong Or Weak.
From www.alamy.com
Acetic Acid, Structural chemical formula on a white background Stock Acetic Acid Strong Or Weak explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. When we add acetic acid to water, it learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. learn the difference between strong acids and weak acids, how they dissociate in water, and. Acetic Acid Strong Or Weak.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions Acetic Acid Strong Or Weak learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. When we add acetic acid to water, it acetic acid ( ) is an example of a weak acid. learn how to identify strong and weak acids and bases with definitions and examples. explore the. Acetic Acid Strong Or Weak.
From chemnotcheem.com
Definition and properties of acids O Level Chemistry Notes Acetic Acid Strong Or Weak acetic acid ( ) is an example of a weak acid. When we add acetic acid to water, it The strength of a weak acid is quantified by its acid dissociation constant, value. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. . Acetic Acid Strong Or Weak.
From quizlet.com
Draw the Lewis structure for acetic acid. Quizlet Acetic Acid Strong Or Weak learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. learn why acetic acid is a weak acid based on its acid dissociation constant and equilibrium reaction. When we. Acetic Acid Strong Or Weak.
From preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories Acetic Acid Strong Or Weak Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. explore the topics and concepts of chemistry with interactive flexbooks, simulations, and plix. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. learn how to identify strong and weak acids and bases with definitions and examples. learn why acetic acid is. Acetic Acid Strong Or Weak.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Net Ionic Equation Tessshebaylo Acetic Acid Strong Or Weak The strength of a weak acid is quantified by its acid dissociation constant, value. acetic acid ( ) is an example of a weak acid. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. thus, a weak acid increases the hydronium ion. Acetic Acid Strong Or Weak.
From saylordotorg.github.io
Aqueous AcidBase Equilibriums Acetic Acid Strong Or Weak learn how to identify strong and weak acids and bases with definitions and examples. Acetic acid (\(\ce{ch3co2h}\)) is a weak acid. The strength of a weak acid is quantified by its acid dissociation constant, value. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a. Acetic Acid Strong Or Weak.
From uhighlsu.web.fc2.com
ph of .1m acetic acid Acetic Acid Strong Or Weak Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. When we add acetic acid to water, it learn how to identify strong and weak acids and bases with definitions and examples. learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based. Acetic Acid Strong Or Weak.
From www.researchgate.net
Study of the conductivity of weak electrolyte solutions acetic acid as Acetic Acid Strong Or Weak learn the definitions and examples of strong and weak acids and bases, and how to distinguish them based on their electrolyte. Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. learn how to identify strong and weak acids and bases with definitions and examples. learn why acetic acid. Acetic Acid Strong Or Weak.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry Atoms First Acetic Acid Strong Or Weak Find out the 7 common strong acids and their properties, and how to measure their acidity with ph. thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid). learn why acetic acid is a weak acid based on its acid dissociation constant and. Acetic Acid Strong Or Weak.