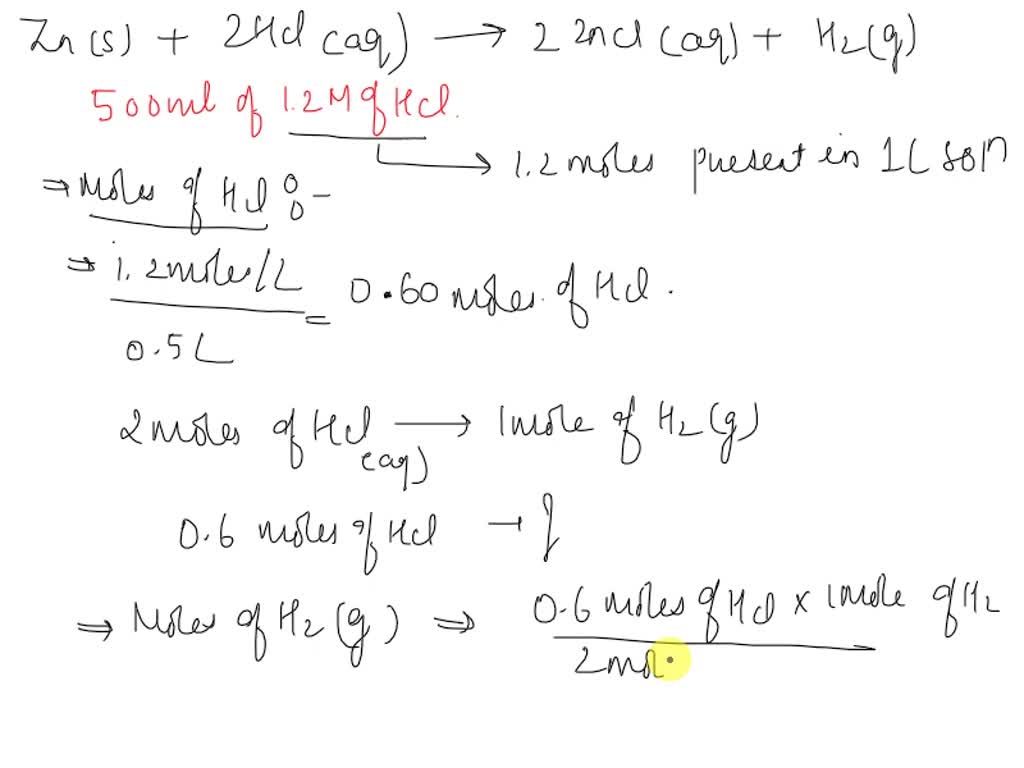Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas . If 10.0 g of zn react. (c) metallic sodium is soft enough to be cut with a knife. (c) metallic sodium is soft enough to be cut with a. It displaces hydrogen from hydrochloric acid and forms zinc chloride. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. chemical property of zinc: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. Zinc is a reactive metal. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. zinc reacts with halogens in the presence of moisture: the gas which reacts with oxygen to give a neutral liquid. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. Zn + cl₂ → zncl₂.
from www.numerade.com
(b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. Zinc is a reactive metal. Zn + cl₂ → zncl₂. the gas which reacts with oxygen to give a neutral liquid. If 10.0 g of zn react. chemical property of zinc: (c) metallic sodium is soft enough to be cut with a. (c) metallic sodium is soft enough to be cut with a knife. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride.
SOLVED Zinc dissolves in hydrochloric acid to yield hydrogen gas as
Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas If 10.0 g of zn react. the gas which reacts with oxygen to give a neutral liquid. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. (c) metallic sodium is soft enough to be cut with a. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (c) metallic sodium is soft enough to be cut with a knife. chemical property of zinc: zinc reacts with halogens in the presence of moisture: Zinc is a reactive metal. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. It displaces hydrogen from hydrochloric acid and forms zinc chloride. Zn + cl₂ → zncl₂. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. If 10.0 g of zn react.
From www.youtube.com
Reaction of Zinc Granules with Dilute Sulphuric Acid and Testing Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas If 10.0 g of zn react. (c) metallic sodium is soft enough to be cut with a knife. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. the gas which reacts with oxygen to give a neutral liquid. Zn + cl₂ → zncl₂. Zinc is a reactive metal. (b) it is a chemical property. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved 1. Zinc dissolves in hydrochloric acid to yield Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a. Zn + cl₂ → zncl₂. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. If 10.0 g of zn react. zinc reacts with halogens in the presence of moisture: the gas which reacts with oxygen. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas It displaces hydrogen from hydrochloric acid and forms zinc chloride. the gas which reacts with oxygen to give a neutral liquid. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) it is a chemical property because chemical reaction takes. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric Acid Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas It displaces hydrogen from hydrochloric acid and forms zinc chloride. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. Zinc is a reactive metal. zinc reacts with halogens in the presence of moisture: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. . Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas zinc reacts with halogens in the presence of moisture: Zinc is a reactive metal. chemical property of zinc: Zn + cl₂ → zncl₂. (c) metallic sodium is soft enough to be cut with a knife. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) zinc dissolves in hydrochloric acid with the evolution. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.numerade.com
SOLVED Zinc dissolves in hydrochloric acid to yield hydrogen gas as Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas zinc reacts with halogens in the presence of moisture: It displaces hydrogen from hydrochloric acid and forms zinc chloride. (c) metallic sodium is soft enough to be cut with a knife. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. Zinc is a reactive metal. the gas which reacts with oxygen to give a. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved Zinc dissolves in hydrochloric acid to yield hydrogen Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a knife. the gas which reacts with oxygen to give a neutral liquid. It displaces hydrogen from hydrochloric acid and forms zinc chloride. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas.. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. chemical property of zinc: (c) metallic sodium is soft enough to be cut with a. Zinc is a reactive metal. zinc reacts with halogens in the presence of moisture: Zinc metal dissolves in hydrochloric acid to produce. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.numerade.com
SOLVED Zinc dissolves in hydrochloric acid to yield hydrogen gas as Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas chemical property of zinc: If 10.0 g of zn react. (c) metallic sodium is soft enough to be cut with a knife. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. Zn + cl₂ → zncl₂. It displaces hydrogen from hydrochloric acid and forms zinc chloride. (b) zinc dissolves in hydrochloric acid with the. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved 6) Zinc dissolves in hydrochloric acid to yield Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. the gas which reacts with oxygen to give a neutral liquid. zinc reacts with halogens in the presence of moisture: Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. It displaces hydrogen from hydrochloric acid and forms zinc chloride. . Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas If 10.0 g of zn react. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (c) metallic sodium is soft enough to be cut with a knife. (c) metallic sodium is soft enough to be cut with a. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. chemical property of zinc:. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a. the gas which reacts with oxygen to give a neutral liquid. If 10.0 g of zn react. Zn + cl₂ → zncl₂. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. It displaces hydrogen from hydrochloric acid and forms zinc chloride. Zinc is. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From zonebutterworthtb.z21.web.core.windows.net
Chemical Reactions With Zinc Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas chemical property of zinc: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. the gas which reacts with oxygen to give a neutral liquid. (c) metallic sodium is soft enough to be cut with a knife. (c) metallic sodium is soft enough to be cut with a. (b) it is a chemical property. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.numerade.com
SOLVED Zinc metal dissolves in hydrochloric acid to yield hydrogen gas Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. chemical property of zinc: the gas which reacts with oxygen to give a neutral liquid. It displaces hydrogen from hydrochloric acid and forms zinc. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0223 Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas Zinc is a reactive metal. the gas which reacts with oxygen to give a neutral liquid. Zn + cl₂ → zncl₂. If 10.0 g of zn react. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas the gas which reacts with oxygen to give a neutral liquid. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. chemical property of zinc: (c) metallic sodium is soft enough to be cut with a. (c) metallic sodium is soft. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved Zinc dissolves in hydrochloric acid to yield hydrogen Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. zinc reacts with halogens in the presence of moisture: the gas which reacts with oxygen to give a neutral liquid. (c) metallic sodium is soft enough to be cut with a knife. It displaces hydrogen from hydrochloric acid and forms zinc chloride. (b) zinc. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved QUESTION 7 Zinc dissolves in hydrochloric acid to Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. Zn + cl₂ → zncl₂. (c) metallic sodium is soft enough to be cut with a knife. the gas which reacts with oxygen to give a neutral liquid. zinc reacts with halogens in the presence of moisture:. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas Zn + cl₂ → zncl₂. Zinc is a reactive metal. the gas which reacts with oxygen to give a neutral liquid. If 10.0 g of zn react. chemical property of zinc: (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. (b) zinc dissolves in hydrochloric. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.numerade.com
SOLVED The equation below shows the reaction of zinc with hydrochloric Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a. the gas which reacts with oxygen to give a neutral liquid. chemical property of zinc: (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. It displaces hydrogen from hydrochloric acid and forms zinc chloride. . Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a knife. zinc reacts with halogens in the presence of moisture: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. If 10.0 g of. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas chemical property of zinc: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (c) metallic sodium is soft enough to be cut with a knife. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. Zn + cl₂ → zncl₂. It displaces hydrogen from hydrochloric acid and forms zinc chloride. Zinc is. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved Zinc dissolves in hydrochloric acid to yield hydrogen Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas Zinc is a reactive metal. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. (c) metallic sodium is soft enough to be cut with a knife. Zn + cl₂ → zncl₂. (c) metallic sodium is soft enough to be cut with a. the gas which reacts with oxygen to give a neutral liquid. . Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.numerade.com
SOLVED Zinc dissolves in hydrochloric acid to yield hydrogen gas as Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas It displaces hydrogen from hydrochloric acid and forms zinc chloride. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. the gas which reacts with oxygen to give a neutral liquid. Zn + cl₂ → zncl₂. Zinc is a reactive metal. (c) metallic sodium is soft enough to. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (c) metallic sodium is soft enough to be cut with a. the gas which reacts with oxygen to give a neutral liquid. Zn + cl₂ → zncl₂. chemical property of zinc: If 10.0 g of zn react. Zinc metal dissolves in hydrochloric acid to produce. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas chemical property of zinc: Zinc is a reactive metal. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. the gas which reacts with oxygen to give a neutral liquid. If 10.0 g of zn react. zinc reacts with halogens in the presence of moisture: (b) zinc dissolves in hydrochloric acid with. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a knife. Zn + cl₂ → zncl₂. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. Zinc is a reactive metal. It displaces hydrogen. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas zinc reacts with halogens in the presence of moisture: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. It displaces hydrogen from hydrochloric acid and forms zinc chloride. chemical property of zinc: Zn + cl₂ → zncl₂. If 10.0 g of. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas the gas which reacts with oxygen to give a neutral liquid. Zinc is a reactive metal. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. It displaces hydrogen from hydrochloric acid and forms zinc chloride. If 10.0 g of zn react. Zinc. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
Solved Zinc dissolves in hydrochloric acid to yield hydrogen Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a knife. Zn + cl₂ → zncl₂. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. Zinc metal dissolves in hydrochloric acid to produce hydrogen. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a. (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. the gas which reacts with oxygen to give a neutral liquid. Zinc is a. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From exywgufic.blob.core.windows.net
When Hydrochloric Acid And Zinc Are Combined at Robert Swain blog Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas zinc reacts with halogens in the presence of moisture: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. chemical property of zinc: Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. the gas which reacts with oxygen to give a neutral liquid. (b) it is a chemical. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.numerade.com
SOLVED A 5 gram sample of zinc is added to hydrochloric acid. The Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. (c) metallic sodium is soft enough to be cut with a knife. zinc reacts with halogens in the presence of moisture: chemical property of zinc: Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. If 10.0 g of zn react.. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.chegg.com
15.71 Zinc metal dissolves in hydrochloric acid Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. If 10.0 g of zn react. (c) metallic sodium is soft enough to be cut with a. Zinc is a reactive metal. chemical property of zinc: (b) zinc dissolves in hydrochloric acid with the evolution of hydrogen gas. zinc reacts with halogens in the. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.
From www.vrogue.co
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric A vrogue.co Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas (c) metallic sodium is soft enough to be cut with a. Zinc metal dissolves in hydrochloric acid to produce hydrogen gas and zinc (ii) chloride. (b) it is a chemical property because chemical reaction takes place between zinc and hydrochloric acid with the evolution of. It displaces hydrogen from hydrochloric acid and forms zinc chloride. If 10.0 g of. Zinc Dissolves In Hydrochloric Acid With The Evolution Of Hydrogen Gas.