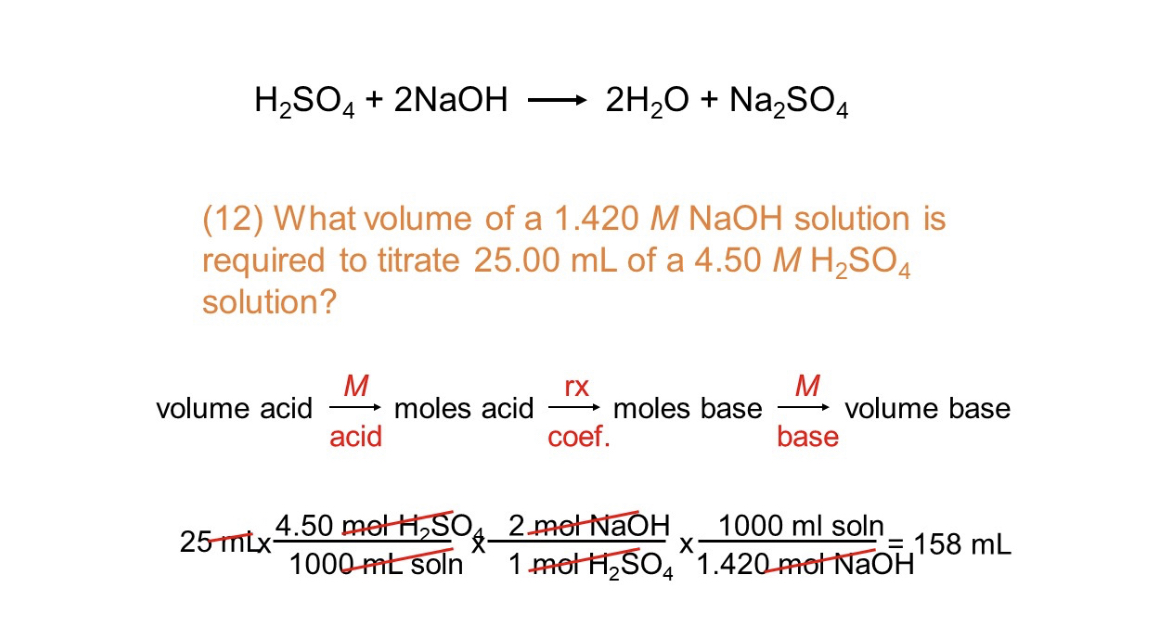Titration Of Ammonia With H2So4 . Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). This application describes the distillation of samples that contain ammonia and the subsequent determination using the. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. The ammonium ion is slightly. Our titration calculator will help you never have to ask how do i calculate titrations? again. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve.
from www.chegg.com
The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is slightly. Our titration calculator will help you never have to ask how do i calculate titrations? again. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. This application describes the distillation of samples that contain ammonia and the subsequent determination using the.
Solved H2SO4+2NaOH 2H2O+Na2SO4 (12) What volume of a
Titration Of Ammonia With H2So4 For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. Our titration calculator will help you never have to ask how do i calculate titrations? again. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is slightly. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed.
From www.numerade.com
SOLVED . A 0.9092 g sample of wheat flour was analysed by the Kjeldahl Titration Of Ammonia With H2So4 The ammonium ion is slightly. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. Generally, cations. Titration Of Ammonia With H2So4.
From www.chegg.com
Solved 26. Ammonia (NH3) and sulfuric acid (H2SO4) react Titration Of Ammonia With H2So4 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The ammonium ion is slightly. Our titration calculator will help you never have to ask how do i calculate titrations? again. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. Generally, cations should. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED 31. Which represents the titration of ammonia with HCl? (A) NH3 Titration Of Ammonia With H2So4 For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. This application describes the distillation of samples that contain ammonia and. Titration Of Ammonia With H2So4.
From www.chegg.com
Solved Consider the titration of ammonia solution with Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). This application describes the distillation of samples that contain ammonia and the subsequent determination using the. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. For. Titration Of Ammonia With H2So4.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Of Ammonia With H2So4 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Our titration calculator will help you never have to ask how do i calculate titrations? again. The ammonium ion is slightly. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. Generally, cations should. Titration Of Ammonia With H2So4.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration Of Ammonia With H2So4 Our titration calculator will help you never have to ask how do i calculate titrations? again. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following. Titration Of Ammonia With H2So4.
From www.coursehero.com
[Solved] For the titration of sulfuric acid (H2SO4) with sodium hyd Titration Of Ammonia With H2So4 On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is. Titration Of Ammonia With H2So4.
From www.chegg.com
Solved Look at the titration graphs below. Determine which Titration Of Ammonia With H2So4 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Our titration calculator will help you never have to ask how do i calculate titrations? again. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). For. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVEDCalculate the volume in milliliters of a 1.420 M NaOH solution Titration Of Ammonia With H2So4 The ammonium ion is slightly. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). This application describes the distillation of samples that contain ammonia and the subsequent determination using the. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric. Titration Of Ammonia With H2So4.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Of Ammonia With H2So4 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. For example, if you titrate ammonia solution. Titration Of Ammonia With H2So4.
From www.chegg.com
Solved H2SO4+2NaOH 2H2O+Na2SO4 (12) What volume of a Titration Of Ammonia With H2So4 On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. This application describes the distillation of samples that contain ammonia and. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED 0.53g of acetanilide was subjected to Kjeldahl determination Titration Of Ammonia With H2So4 For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is slightly.. Titration Of Ammonia With H2So4.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Of Ammonia With H2So4 On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. The ammonium ion is slightly. For example, if you titrate ammonia. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVEDWhich graph best represents the titration of ammonia with Titration Of Ammonia With H2So4 This application describes the distillation of samples that contain ammonia and the subsequent determination using the. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The ammonium ion is slightly. On a molecular. Titration Of Ammonia With H2So4.
From www.chegg.com
Solved 1 The titration of 0.050 M aqueous ammonia with Titration Of Ammonia With H2So4 For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. Our titration calculator will help you never have to ask how do i calculate titrations? again. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following. Titration Of Ammonia With H2So4.
From www.vrogue.co
Solved Titration Of Ammonia With Hcl This Graph Shows vrogue.co Titration Of Ammonia With H2So4 This application describes the distillation of samples that contain ammonia and the subsequent determination using the. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as. Titration Of Ammonia With H2So4.
From www.vrogue.co
Solved Titration Of Ammonia With Hcl This Graph Shows vrogue.co Titration Of Ammonia With H2So4 Our titration calculator will help you never have to ask how do i calculate titrations? again. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. On a molecular level, describe what is happening during the titration of. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED A solution of sulfuric acid with molarity 0.38 M is used to Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is slightly. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. Our titration calculator will help you never have to ask how do i calculate titrations? again.. Titration Of Ammonia With H2So4.
From www.mdpi.com
Energies Free FullText Quantification Methodology of Ammonia Titration Of Ammonia With H2So4 On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). For example, if you. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED Consider the titration of sulfuric acid with sodium hydroxide Titration Of Ammonia With H2So4 For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following. Titration Of Ammonia With H2So4.
From www.chegg.com
Solved Titration of ammonia with HCl This graph shows the Titration Of Ammonia With H2So4 On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. The example below demonstrates the technique to solve a titration problem. Titration Of Ammonia With H2So4.
From byjus.com
In the Kjeldahl's method for estimation of nitrogen present in a soil Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is slightly. Our titration calculator will help you never have to ask how do i calculate titrations? again. This application describes the distillation of samples that contain ammonia and the subsequent determination using the.. Titration Of Ammonia With H2So4.
From www.slideserve.com
PPT Ammonia Titration Laboratory Activity The Problem PowerPoint Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. The ammonium ion is. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED VII Answer the following questions concerning the determination Titration Of Ammonia With H2So4 Our titration calculator will help you never have to ask how do i calculate titrations? again. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED A 0.9325 g sample of wheat flour was analyzed using the Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. This. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED Part E Ammonia. A 0.467 g sample known to contain ammonia is Titration Of Ammonia With H2So4 On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. The example below demonstrates the technique to solve a titration problem. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED A0.256 M of H2SO4 was used to titrate 55.0 ml of NaOH 24.5 ml Titration Of Ammonia With H2So4 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. The. Titration Of Ammonia With H2So4.
From signalticket9.pythonanywhere.com
Great Ammonia Plus Sulphuric Acid Balancing Equations Practice Problems Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is slightly. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. This application describes the distillation of samples that contain ammonia and the subsequent determination using the.. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED 31. Which represents the titration of ammonia with HCl? (A) NH3 Titration Of Ammonia With H2So4 For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. The ammonium ion is slightly. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. Our titration calculator will help you. Titration Of Ammonia With H2So4.
From www.coursehero.com
[Solved] A 25.00 ml volume of sulfuric acid was titrated with a Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). The ammonium ion is slightly. Our titration calculator will help you never have to ask how do i calculate titrations? again. On a molecular level, describe what is happening during the titration of a weak base, such. Titration Of Ammonia With H2So4.
From www.chegg.com
Solved Part B. Titration of Ammonia with HCI 1. Use the Titration Of Ammonia With H2So4 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. This application describes the distillation of samples that contain ammonia and the subsequent determination using the. The ammonium ion is slightly. Generally, cations should. Titration Of Ammonia With H2So4.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Of Ammonia With H2So4 Our titration calculator will help you never have to ask how do i calculate titrations? again. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). On. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED Question 6 The graphs labeled (I) through (III) show acidbase Titration Of Ammonia With H2So4 Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). For example, if you titrate ammonia solution with hydrochloric acid, you would get ammonium chloride formed. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Our. Titration Of Ammonia With H2So4.
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced Titration Of Ammonia With H2So4 This application describes the distillation of samples that contain ammonia and the subsequent determination using the. On a molecular level, describe what is happening during the titration of a weak base, such as ammonia, with a strong acid, such as hcl, at the following points along the titration curve. Our titration calculator will help you never have to ask how. Titration Of Ammonia With H2So4.
From www.numerade.com
SOLVED The fertilizer ammonium sulfate is prepared by the reaction Titration Of Ammonia With H2So4 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The ammonium ion is slightly. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity (table 4.2 of unit 4). For example, if you titrate ammonia solution with hydrochloric acid, you would. Titration Of Ammonia With H2So4.