What Is The Atomic Shell Hypothesis . One of the most successful and simple to. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. Many models describe the way protons and neutrons are arranged inside a nucleus. The atomic shell model explains the structure of atoms. One such model is the shell model, which accounts for many features of the nuclear energy levels. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. The model described the atom as a tiny, dense, positively charged. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. Nucleons are added to shells which increase with energy that orbit around a central. According to this model, the motion of each nucleon is governed by the average.
from stock.adobe.com
Many models describe the way protons and neutrons are arranged inside a nucleus. According to this model, the motion of each nucleon is governed by the average. The model described the atom as a tiny, dense, positively charged. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. One such model is the shell model, which accounts for many features of the nuclear energy levels. Nucleons are added to shells which increase with energy that orbit around a central. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. The atomic shell model explains the structure of atoms. One of the most successful and simple to.
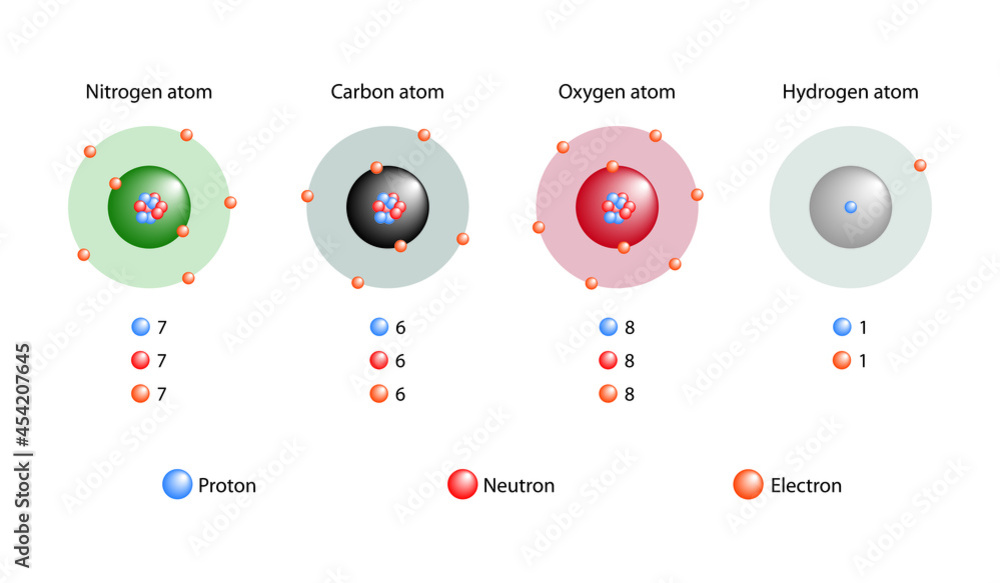
Atomic elements showing the nucleus and shells, numbers of electrons
What Is The Atomic Shell Hypothesis In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. According to this model, the motion of each nucleon is governed by the average. The atomic shell model explains the structure of atoms. The model described the atom as a tiny, dense, positively charged. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. Nucleons are added to shells which increase with energy that orbit around a central. One such model is the shell model, which accounts for many features of the nuclear energy levels. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. Many models describe the way protons and neutrons are arranged inside a nucleus. One of the most successful and simple to.
From www.slideserve.com
PPT The Atom Hypothesis PowerPoint Presentation, free download ID What Is The Atomic Shell Hypothesis The model described the atom as a tiny, dense, positively charged. Many models describe the way protons and neutrons are arranged inside a nucleus. One of the most successful and simple to. According to this model, the motion of each nucleon is governed by the average. Nucleons are added to shells which increase with energy that orbit around a central.. What Is The Atomic Shell Hypothesis.
From mammothmemory.net
Electrons orbit the nucleus of an atom orbits are called she What Is The Atomic Shell Hypothesis In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. The model described the atom as a tiny, dense, positively charged. Nucleons are added to shells which increase with energy that orbit around a central. One of the most successful and simple to. The. What Is The Atomic Shell Hypothesis.
From www.linstitute.net
IB DP Chemistry HL复习笔记2.1.6 Energy Levels & Sublevels翰林国际教育 What Is The Atomic Shell Hypothesis According to this model, the motion of each nucleon is governed by the average. One of the most successful and simple to. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. In the atomic shell model, the central potential around which the nucleus. What Is The Atomic Shell Hypothesis.
From www.pinterest.com
Atoms, shells ,Subshells and Orbitals Atom, Submarine, Shells What Is The Atomic Shell Hypothesis Many models describe the way protons and neutrons are arranged inside a nucleus. The model described the atom as a tiny, dense, positively charged. Nucleons are added to shells which increase with energy that orbit around a central. One of the most successful and simple to. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic. What Is The Atomic Shell Hypothesis.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Atomic Shell Hypothesis In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. One of the most successful and simple to. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. The model described the atom as a tiny,. What Is The Atomic Shell Hypothesis.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.1.6 Electronic Structure翰林国际教育 What Is The Atomic Shell Hypothesis In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. The model described the atom as a tiny, dense, positively charged. The atomic shell model explains the structure of atoms. According to this model, the motion of each nucleon is governed by the average. One such model is the shell model, which accounts for. What Is The Atomic Shell Hypothesis.
From stock.adobe.com
Atomic elements showing the nucleus and shells, numbers of electrons What Is The Atomic Shell Hypothesis According to this model, the motion of each nucleon is governed by the average. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. In the. What Is The Atomic Shell Hypothesis.
From www.myxxgirl.com
Teori Model Atom Modern My XXX Hot Girl What Is The Atomic Shell Hypothesis Nucleons are added to shells which increase with energy that orbit around a central. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. According to this model,. What Is The Atomic Shell Hypothesis.
From www.shutterstock.com
5,046 Electrons Shell Images, Stock Photos & Vectors Shutterstock What Is The Atomic Shell Hypothesis The model described the atom as a tiny, dense, positively charged. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. According to this model, the motion of. What Is The Atomic Shell Hypothesis.
From chyscience.blogspot.com
Chemical Science Electronic structure of an atom What Is The Atomic Shell Hypothesis According to this model, the motion of each nucleon is governed by the average. One such model is the shell model, which accounts for many features of the nuclear energy levels. The atomic shell model explains the structure of atoms. Nucleons are added to shells which increase with energy that orbit around a central. The negatively charged fundamental particles which. What Is The Atomic Shell Hypothesis.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor What Is The Atomic Shell Hypothesis The model described the atom as a tiny, dense, positively charged. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. Many models describe the way protons and neutrons are arranged inside a nucleus. The negatively charged fundamental particles which are known as electrons. What Is The Atomic Shell Hypothesis.
From brainly.in
draw a diagram of bohrs model of an atom showing four energy shells What Is The Atomic Shell Hypothesis Nucleons are added to shells which increase with energy that orbit around a central. One of the most successful and simple to. The model described the atom as a tiny, dense, positively charged. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. In. What Is The Atomic Shell Hypothesis.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Is The Atomic Shell Hypothesis According to this model, the motion of each nucleon is governed by the average. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. Nucleons are added to. What Is The Atomic Shell Hypothesis.
From www.slideserve.com
PPT 11 The Atomic Nature of Matter PowerPoint Presentation, free What Is The Atomic Shell Hypothesis The atomic shell model explains the structure of atoms. The model described the atom as a tiny, dense, positively charged. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. One such model is the shell model, which accounts for many features of the. What Is The Atomic Shell Hypothesis.
From www.britannica.com
Atom Proton, Neutron, Nucleus Britannica What Is The Atomic Shell Hypothesis In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. Many models describe the way protons and neutrons are arranged inside a nucleus. The atomic shell model explains the structure of atoms. One of the most successful and simple to. Nucleons are added to. What Is The Atomic Shell Hypothesis.
From www.youtube.com
What are shells,subshells and orbitals? Difference between shells What Is The Atomic Shell Hypothesis Nucleons are added to shells which increase with energy that orbit around a central. According to this model, the motion of each nucleon is governed by the average. The atomic shell model explains the structure of atoms. One of the most successful and simple to. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse. What Is The Atomic Shell Hypothesis.
From scienceready.com.au
de Broglie's Matter Waves & Supporting Evidence HSC Physics Science What Is The Atomic Shell Hypothesis One of the most successful and simple to. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. In the atomic shell model, the central potential. What Is The Atomic Shell Hypothesis.
From socratic.org
What is the difference between electron shells and electron orbitals What Is The Atomic Shell Hypothesis One of the most successful and simple to. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. The atomic shell model explains the structure of atoms. One such model is the shell model, which accounts for many features of the nuclear energy levels.. What Is The Atomic Shell Hypothesis.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Atomic Shell Hypothesis One of the most successful and simple to. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. Nucleons are added to shells which increase with energy that orbit around a central. In the atomic shell model, the central potential around which the nucleus. What Is The Atomic Shell Hypothesis.
From socratic.org
Question e9926 Socratic What Is The Atomic Shell Hypothesis The model described the atom as a tiny, dense, positively charged. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. One such model is the shell model, which accounts for many features of the nuclear energy levels. Nucleons are added to shells which increase with energy that orbit around a central. One of. What Is The Atomic Shell Hypothesis.
From www.youtube.com
What is the Difference between Shell and Subshell Chemistry Class 9 What Is The Atomic Shell Hypothesis In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. Many models describe the way protons and neutrons are arranged inside a nucleus. The atomic shell model explains the structure of atoms. One of the most successful and simple to. The negatively charged fundamental particles which are known as electrons are considered to occupy. What Is The Atomic Shell Hypothesis.
From byjus.com
what is the sequence of shell , subshell, orbitals , orbits , electrons What Is The Atomic Shell Hypothesis The atomic shell model explains the structure of atoms. One of the most successful and simple to. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. According to this model, the motion of each nucleon is governed by the average. The negatively charged. What Is The Atomic Shell Hypothesis.
From bmp-level.blogspot.com
What Is An Electron Shell Definition bmplevel What Is The Atomic Shell Hypothesis In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. The model described the atom as a tiny, dense, positively charged. One of the most successful and simple to. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. One such model is the shell model,. What Is The Atomic Shell Hypothesis.
From www.scienceabc.com
Octet Rule Definition, Explanation, Exceptions And Examples What Is The Atomic Shell Hypothesis According to this model, the motion of each nucleon is governed by the average. One of the most successful and simple to. The atomic shell model explains the structure of atoms. Nucleons are added to shells which increase with energy that orbit around a central. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse. What Is The Atomic Shell Hypothesis.
From mucholderthen.tumblr.com
Science Visualized • A BRIEF TIMELINE OF ATOMIC THEORY The idea that... What Is The Atomic Shell Hypothesis Nucleons are added to shells which increase with energy that orbit around a central. Many models describe the way protons and neutrons are arranged inside a nucleus. One such model is the shell model, which accounts for many features of the nuclear energy levels. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to. What Is The Atomic Shell Hypothesis.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Atomic Shell Hypothesis In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. Many models describe the way protons and neutrons are arranged inside a nucleus. The model described the atom. What Is The Atomic Shell Hypothesis.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Atomic Structure What Is The Atomic Shell Hypothesis One such model is the shell model, which accounts for many features of the nuclear energy levels. The model described the atom as a tiny, dense, positively charged. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. Nucleons are added to shells which increase with energy that orbit around a central. One of. What Is The Atomic Shell Hypothesis.
From www.slideserve.com
PPT Chapter 12 Atoms & the Periodic Table PowerPoint Presentation What Is The Atomic Shell Hypothesis Nucleons are added to shells which increase with energy that orbit around a central. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. Many models describe the way protons and neutrons are arranged inside a nucleus. One such model is the shell model,. What Is The Atomic Shell Hypothesis.
From mmerevise.co.uk
Atoms Questions and Revision MME What Is The Atomic Shell Hypothesis The model described the atom as a tiny, dense, positively charged. One such model is the shell model, which accounts for many features of the nuclear energy levels. One of the most successful and simple to. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. The atomic shell model explains the. What Is The Atomic Shell Hypothesis.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube What Is The Atomic Shell Hypothesis One of the most successful and simple to. According to this model, the motion of each nucleon is governed by the average. In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. Nucleons are added to shells which increase with energy that orbit around a central. Many models describe the way protons and neutrons. What Is The Atomic Shell Hypothesis.
From www.worksheetsplanet.com
Dalton's Atomic Theory What Is The Atomic Shell Hypothesis One such model is the shell model, which accounts for many features of the nuclear energy levels. Nucleons are added to shells which increase with energy that orbit around a central. One of the most successful and simple to. The atomic shell model explains the structure of atoms. The model described the atom as a tiny, dense, positively charged. According. What Is The Atomic Shell Hypothesis.
From www.slideserve.com
PPT Review of Atomic Theory PowerPoint Presentation, free download What Is The Atomic Shell Hypothesis In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. One of the most successful and simple to. The atomic shell model explains the structure of atoms. One such model is the shell model, which accounts for many features of the nuclear energy levels. Many models describe the way protons and neutrons are arranged. What Is The Atomic Shell Hypothesis.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? What Is The Atomic Shell Hypothesis Many models describe the way protons and neutrons are arranged inside a nucleus. The model described the atom as a tiny, dense, positively charged. Nucleons are added to shells which increase with energy that orbit around a central. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. In the atomic shell. What Is The Atomic Shell Hypothesis.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 What Is The Atomic Shell Hypothesis According to this model, the motion of each nucleon is governed by the average. In 1913 bohr proposed his quantized shell model of the atom (see bohr atomic model) to explain how electrons can have stable orbits around the nucleus. One such model is the shell model, which accounts for many features of the nuclear energy levels. The negatively charged. What Is The Atomic Shell Hypothesis.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is The Atomic Shell Hypothesis In the atomic shell model, the central potential around which the nucleus generates the electrons orbit. The model described the atom as a tiny, dense, positively charged. Nucleons are added to shells which increase with energy that orbit around a central. The negatively charged fundamental particles which are known as electrons are considered to occupy diffuse shells in the. One. What Is The Atomic Shell Hypothesis.