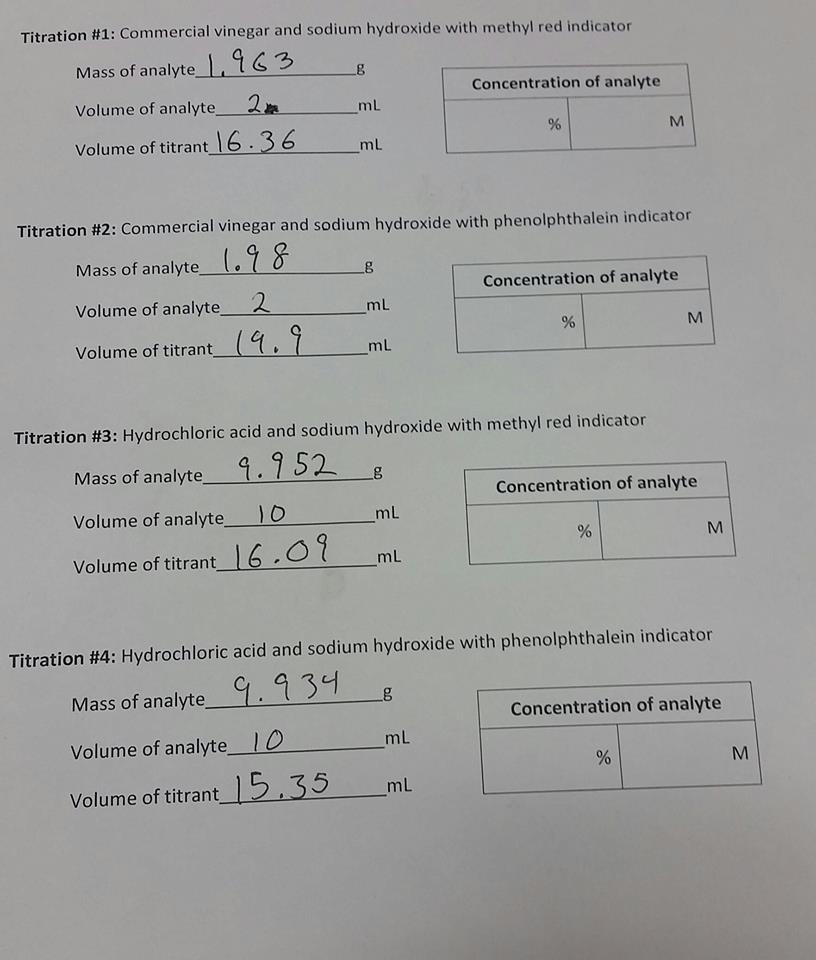
Vinegar And Sodium Hydroxide Formula . here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. An analytical procedure involving a chemical. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar.
from www.chegg.com
here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. An analytical procedure involving a chemical. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. the reaction equation is:
Solved Commercial vinegar and sodium hydroxide with methyl
Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. An analytical procedure involving a chemical.
From www.coursehero.com
[Solved] Using a 0.2564 M solution of sodium hydroxide, a vinegar Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. the reaction equation is: An analytical procedure involving a chemical. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one. Vinegar And Sodium Hydroxide Formula.
From www.thinkswap.com
Titration Practical, Vinegar and Sodium Hydroxide. Chemistry Year Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is. Vinegar And Sodium Hydroxide Formula.
From www.youtube.com
write the formula for sodium hydroxide sodium hydroxide Formula Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh). Vinegar And Sodium Hydroxide Formula.
From www.chegg.com
Solved Commercial vinegar and sodium hydroxide with methyl Vinegar And Sodium Hydroxide Formula An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. the reaction equation is: regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required. Vinegar And Sodium Hydroxide Formula.
From exolwslgk.blob.core.windows.net
Vinegar Chemical Formula at Dorothy Lazzaro blog Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is. Vinegar And Sodium Hydroxide Formula.
From www.vectorstock.com
Naoh sodium hydroxide molecule Royalty Free Vector Image Vinegar And Sodium Hydroxide Formula the reaction equation is: regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and. Vinegar And Sodium Hydroxide Formula.
From www.chegg.com
Solved Calculate the combined mass of the two reactants. 5 Vinegar And Sodium Hydroxide Formula An analytical procedure involving a chemical. the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED The distinctive odor of vinegar is due to acetic acid, CH3COOH Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED A solution of sodium hydroxide (NaOH) was standardized against Vinegar And Sodium Hydroxide Formula the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each. Vinegar And Sodium Hydroxide Formula.
From gbu-presnenskij.ru
Sodium Hydroxide Skeletal Formula Royalty Free Vector Image, 53 OFF Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. An. Vinegar And Sodium Hydroxide Formula.
From schoolworkhelper.net
Titration of Vinegar Lab Report Sodium Hydroxide and Vinegar Vinegar And Sodium Hydroxide Formula the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED Titlewithtopic Titration of Vinegar Solution with Sodium Hydroxide Vinegar And Sodium Hydroxide Formula regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide. Vinegar And Sodium Hydroxide Formula.
From mavink.com
Chemical Formula Of Sodium Hydroxide Vinegar And Sodium Hydroxide Formula the reaction equation is: Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. here, the titrant is an aqueous solution of. Vinegar And Sodium Hydroxide Formula.
From mavink.com
Acetic Acid And Sodium Hydroxide Reaction Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. An analytical procedure involving a chemical. the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. regardless of how the equation is written, one mole of. Vinegar And Sodium Hydroxide Formula.
From www.chegg.com
Solved Data Table 1 Titration of Vinegar with Sodium Vinegar And Sodium Hydroxide Formula the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. An analytical procedure involving a chemical. Determine the number of moles of sodium hydroxide required to titrate. Vinegar And Sodium Hydroxide Formula.
From www.vectorstock.com
Sodium hydroxide skeletal formula Royalty Free Vector Image Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity. Vinegar And Sodium Hydroxide Formula.
From www.studocu.com
Standardization of Na OH solution Lab report Chem Standardization of Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. An analytical procedure involving a chemical. the reaction equation is: Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED PHASE 4 Titrate vinegar sample Complete the following steps Vinegar And Sodium Hydroxide Formula An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one mole of acetic acid in. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED Sodium hydroxide (base) and acetic acid react in a 11 molar Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. the reaction equation is: An analytical procedure involving a chemical. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of. Vinegar And Sodium Hydroxide Formula.
From www.scribd.com
8_Titration of Vinegar Titration Sodium Hydroxide Vinegar And Sodium Hydroxide Formula regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. the reaction equation is: Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. An analytical procedure involving a chemical. here, the titrant is an aqueous. Vinegar And Sodium Hydroxide Formula.
From www.chegg.com
TITRATION OF VINEGAR with SODIUM HYDROXIDE 5. Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. the reaction equation is: regardless of how the equation is written, one mole of acetic acid in the. Vinegar And Sodium Hydroxide Formula.
From pharmabeej.com
What Is Molecular Formula Of Sodium Hydroxide? Pharmabeej Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of. Vinegar And Sodium Hydroxide Formula.
From www.thinkswap.com
AcidBase Titration of Vinegar and Sodium Hydroxide Chemistry Vinegar And Sodium Hydroxide Formula An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required to titrate the vinegar for. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED Suppose you are titrating vinegar, which is an acetic acid Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. An analytical procedure involving a chemical. the reaction equation is: Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED Verify your calculation Standardized NaOH (M) 0.4355 Initial Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. the reaction equation is: An analytical procedure involving a chemical. here, the titrant is an aqueous solution of. Vinegar And Sodium Hydroxide Formula.
From www.youtube.com
Titration Vinegar with Sodium Hydroxide YouTube Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in. Vinegar And Sodium Hydroxide Formula.
From docslib.org
Preparation of Standard Sodium Hydroxide Solution and Titration of a Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. An analytical procedure involving a chemical. the reaction equation is: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. regardless of how the equation is written, one. Vinegar And Sodium Hydroxide Formula.
From shineshower30.gitlab.io
Stunning Nacl H2o Balanced Equation Physics 12 Formula Sheet Pdf Vinegar And Sodium Hydroxide Formula the reaction equation is: An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. here, the titrant is an aqueous solution of. Vinegar And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED Suppose you are titrating vinegar; which is an acetic acid Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. An analytical procedure involving a chemical. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. regardless of how the equation is written, one mole of acetic acid in. Vinegar And Sodium Hydroxide Formula.
From cartoondealer.com
Sodium Hydroxide, Compound, Molecular Structures, 3d Model Vinegar And Sodium Hydroxide Formula the reaction equation is: An analytical procedure involving a chemical. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. Determine the number of moles of sodium hydroxide required to titrate. Vinegar And Sodium Hydroxide Formula.
From www.thinkswap.com
AcidBase Titration of Vinegar and Sodium Hydroxide Chemistry Vinegar And Sodium Hydroxide Formula Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. . Vinegar And Sodium Hydroxide Formula.
From www.chegg.com
Data Table 1 Titration of White Vinegar with Sodium Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. the reaction equation is: regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each. Vinegar And Sodium Hydroxide Formula.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Vinegar And Sodium Hydroxide Formula An analytical procedure involving a chemical. Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh). Vinegar And Sodium Hydroxide Formula.
From www.micoope.com.gt
Titration Of Vinegar With Sodium Hydroxide, 49 OFF Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. regardless of how the equation is written, one mole of acetic acid in the vinegar requires one mole of sodium. . Vinegar And Sodium Hydroxide Formula.
From uhighlsu.web.fc2.com
sodium hydroxide + acetic acid Vinegar And Sodium Hydroxide Formula here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (naoh) and the analyte is vinegar. An analytical procedure involving a chemical. the reaction equation is: Determine the number of moles of sodium hydroxide required to titrate the vinegar for each titration from the known molarity and. here, the titrant is an aqueous solution of. Vinegar And Sodium Hydroxide Formula.