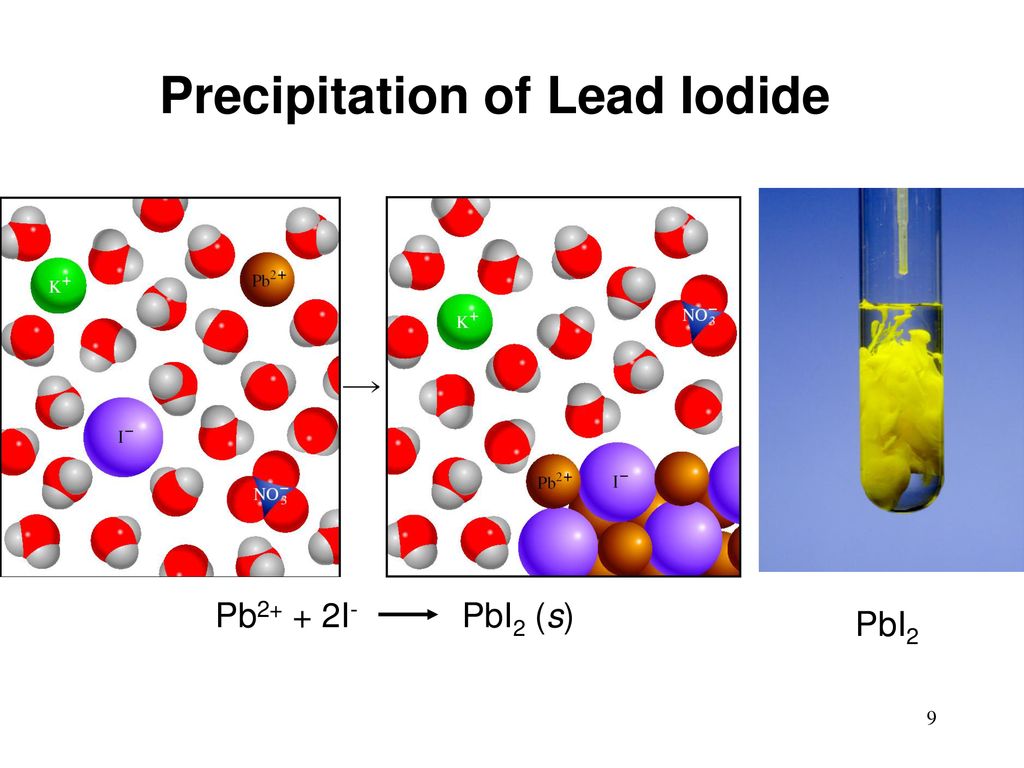
Lead Iodide Ppt . Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). It really is impressive to see. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. Formation of lead (ii) iodide. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). When mixed, two clear solutions produce a dense yellow precipitate. A yellow precipitate will form. Add equal amounts of each solution to the cylinder. To observe the formation of a precipitate.
from slideplayer.com
To observe the formation of a precipitate. When mixed, two clear solutions produce a dense yellow precipitate. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Add equal amounts of each solution to the cylinder. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. It really is impressive to see. A yellow precipitate will form. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. Formation of lead (ii) iodide. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii).
Reactions in Aqueous Solution ppt download
Lead Iodide Ppt When mixed, two clear solutions produce a dense yellow precipitate. It really is impressive to see. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Formation of lead (ii) iodide. A yellow precipitate will form. When mixed, two clear solutions produce a dense yellow precipitate. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Add equal amounts of each solution to the cylinder. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. To observe the formation of a precipitate.
From www.slideserve.com
PPT Chemical Synthesis Lead(II) Iodide PowerPoint Presentation, free Lead Iodide Ppt When mixed, two clear solutions produce a dense yellow precipitate. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. Add equal amounts. Lead Iodide Ppt.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead Iodide Ppt When mixed, two clear solutions produce a dense yellow precipitate. Formation of lead (ii) iodide. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). The tiny. Lead Iodide Ppt.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Iodide Ppt The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. When mixed, two clear solutions produce a dense yellow precipitate. Formation of lead (ii) iodide. A yellow precipitate will form. To observe the formation of a precipitate. Potassium. Lead Iodide Ppt.
From slideplayer.com
Reactions in Aqueous Solution ppt download Lead Iodide Ppt When mixed, two clear solutions produce a dense yellow precipitate. It really is impressive to see. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). A yellow precipitate will form. Formation of lead (ii) iodide. The tiny crystals of lead iodide that form swirl beautifully in the flask. Lead Iodide Ppt.
From www.sciencephoto.com
Lead Iodide Precipitate Forming in Pb(NO3)2 Stock Image A500/0781 Lead Iodide Ppt It really is impressive to see. Formation of lead (ii) iodide. When mixed, two clear solutions produce a dense yellow precipitate. A yellow precipitate will form. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). To observe the formation of a precipitate. Potassium iodide solution is added to. Lead Iodide Ppt.
From www.compoundchem.com
Compound Interest Chemical Reactions Lead Iodide & ‘Golden Rain’ Lead Iodide Ppt Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. To observe the formation of a precipitate. When mixed, two clear solutions produce a dense yellow precipitate. Formation of lead (ii) iodide. A yellow. Lead Iodide Ppt.
From www.slideserve.com
PPT Chemical Synthesis Lead(II) Iodide PowerPoint Presentation, free Lead Iodide Ppt Add equal amounts of each solution to the cylinder. A yellow precipitate will form. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). It really is impressive to see. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to. Lead Iodide Ppt.
From www.slideserve.com
PPT Chemical Synthesis Lead(II) Iodide PowerPoint Presentation, free Lead Iodide Ppt Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. To observe the formation of a precipitate. A yellow precipitate will form. Formation of lead (ii) iodide. Today kicks off with one of my. Lead Iodide Ppt.
From www.slideserve.com
PPT Precipitate Formation Formation of Lead (II) Iodide PowerPoint Lead Iodide Ppt When mixed, two clear solutions produce a dense yellow precipitate. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine. Lead Iodide Ppt.
From www.researchgate.net
2) a) Lead iodide purification setup and stages, and b) produced Lead Iodide Ppt To observe the formation of a precipitate. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant.. Lead Iodide Ppt.
From www.slideserve.com
PPT Precipitate Formation Formation of Lead (II) Iodide PowerPoint Lead Iodide Ppt A yellow precipitate will form. It really is impressive to see. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Formation of lead (ii) iodide. When mixed, two clear solutions produce a dense yellow precipitate. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead. Lead Iodide Ppt.
From www.slideserve.com
PPT Types of Chemical Reactions Single and Double Displacement Lead Iodide Ppt It really is impressive to see. Add equal amounts of each solution to the cylinder. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. When mixed, two clear solutions produce a dense yellow precipitate. Potassium iodide solution. Lead Iodide Ppt.
From www.slideserve.com
PPT Lead(II) IodideWater System PowerPoint Presentation, free Lead Iodide Ppt The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. It really is impressive to see. A yellow precipitate will form. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as. Lead Iodide Ppt.
From depositphotos.com
Lead Iodide Properties Chemical Compound Structure Stock Vector Image Lead Iodide Ppt Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Formation of lead (ii) iodide. When mixed, two clear solutions produce a dense yellow precipitate. It really is impressive to see. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. Add equal amounts. Lead Iodide Ppt.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Ppt Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Formation of lead (ii) iodide. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). To observe the formation of a precipitate. Potassium iodide solution is added. Lead Iodide Ppt.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead Iodide Ppt Formation of lead (ii) iodide. A yellow precipitate will form. Add equal amounts of each solution to the cylinder. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Today kicks off with one of my favourite reactions,. Lead Iodide Ppt.
From www.flickr.com
Lead Iodide Pittsburgh Carnegie Science and Trib Total Med… Flickr Lead Iodide Ppt Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). To observe the formation of a precipitate. Add equal amounts of each solution to the cylinder. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. It really is impressive to see. The. Lead Iodide Ppt.
From www.slideshare.net
Potassium iodide and_lead_nitrate PPT Lead Iodide Ppt The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Add equal amounts of each solution to the cylinder. Formation of lead (ii) iodide. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide. Lead Iodide Ppt.
From www.slideserve.com
PPT Precipitate Formation Formation of Lead (II) Iodide PowerPoint Lead Iodide Ppt To observe the formation of a precipitate. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). A yellow precipitate will form. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere. Lead Iodide Ppt.
From www.pw.live
Lead Iodide Formula, Structure And Molar Mass Lead Iodide Ppt A yellow precipitate will form. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). It really is impressive to see. Add equal amounts of each solution to the cylinder. When mixed, two clear solutions produce a dense yellow precipitate. Lead iodide is a bright yellow solid that. Lead Iodide Ppt.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Lead Iodide Ppt Add equal amounts of each solution to the cylinder. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). To observe the formation of a precipitate. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). A. Lead Iodide Ppt.
From www.slideserve.com
PPT Precipitate Formation Formation of Lead (II) Iodide PowerPoint Lead Iodide Ppt Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. To observe the formation of a. Lead Iodide Ppt.
From slideplayer.com
Reactions in Aqueous Solutions ppt download Lead Iodide Ppt Formation of lead (ii) iodide. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). It really is impressive to see. A yellow precipitate will form. When mixed, two clear solutions produce a dense yellow precipitate. To observe the formation of a precipitate. Today kicks off with one of. Lead Iodide Ppt.
From www.sciencephoto.com
Lead Iodide Precipitate Stock Image C043/5108 Science Photo Library Lead Iodide Ppt Formation of lead (ii) iodide. A yellow precipitate will form. It really is impressive to see. Add equal amounts of each solution to the cylinder. To observe the formation of a precipitate. When mixed, two clear solutions produce a dense yellow precipitate. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and. Lead Iodide Ppt.
From giogzqmmd.blob.core.windows.net
Lead Iodide Potassium Nitrate at Drucilla Foy blog Lead Iodide Ppt The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). When mixed, two clear solutions produce. Lead Iodide Ppt.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Iodide Ppt The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. When mixed, two clear solutions produce a dense yellow precipitate. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine. Lead Iodide Ppt.
From www.slideserve.com
PPT 3. Solutions of sodium iodide and lead nitrate are mixed. I Lead Iodide Ppt It really is impressive to see. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Formation of lead (ii) iodide. When mixed, two clear solutions produce. Lead Iodide Ppt.
From slideplayer.com
Chapter 11 Chemical Reactions. ppt download Lead Iodide Ppt To observe the formation of a precipitate. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. A yellow precipitate will form. The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. Formation. Lead Iodide Ppt.
From alannahminhardy.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide AlannahminHardy Lead Iodide Ppt Add equal amounts of each solution to the cylinder. Formation of lead (ii) iodide. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Today kicks off with one of my favourite reactions, the. Lead Iodide Ppt.
From www.youtube.com
Precipitation Reaction Potassium Iodide KI & Lead (II) Nitrate Pb(NO3)2 Lead Iodide Ppt Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). Formation of lead (ii) iodide. When mixed, two clear solutions produce a dense yellow precipitate. The tiny. Lead Iodide Ppt.
From www.slideserve.com
PPT Onedimensional lead iodide hybrid compound shows a broadband Lead Iodide Ppt Add equal amounts of each solution to the cylinder. Today kicks off with one of my favourite reactions, the ‘golden rain’ demonstration, which involves the synthesis and recrystallisation of lead (ii). To observe the formation of a precipitate. It really is impressive to see. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. Formation. Lead Iodide Ppt.
From www.scribd.com
KSP of Lead Iodide PDF Solubility Physical Sciences Lead Iodide Ppt The tiny crystals of lead iodide that form swirl beautifully in the flask and the concentration gradients combine to generate an effect that looks like the atmosphere of a glittering gas giant. It really is impressive to see. To observe the formation of a precipitate. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment. Lead Iodide Ppt.
From pixels.com
Lead Iodide Precipitate Photograph by Andrew Lambert Photography Pixels Lead Iodide Ppt Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. To observe the formation of a precipitate. A yellow precipitate will form. Formation of lead (ii) iodide. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). Add equal amounts of each solution to. Lead Iodide Ppt.
From www.slideserve.com
PPT Chemical Synthesis Lead(II) Iodide PowerPoint Presentation, free Lead Iodide Ppt Add equal amounts of each solution to the cylinder. When mixed, two clear solutions produce a dense yellow precipitate. A yellow precipitate will form. To observe the formation of a precipitate. It really is impressive to see. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. The tiny crystals of lead iodide that form. Lead Iodide Ppt.
From www.slideserve.com
PPT 3. Solutions of sodium iodide and lead nitrate are mixed. I Lead Iodide Ppt When mixed, two clear solutions produce a dense yellow precipitate. Potassium iodide solution is added to lead nitrate solution, and bright yellow lead iodide precipitates. Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (figure 15.3a). It really is impressive to see. To observe the formation of a precipitate. The. Lead Iodide Ppt.