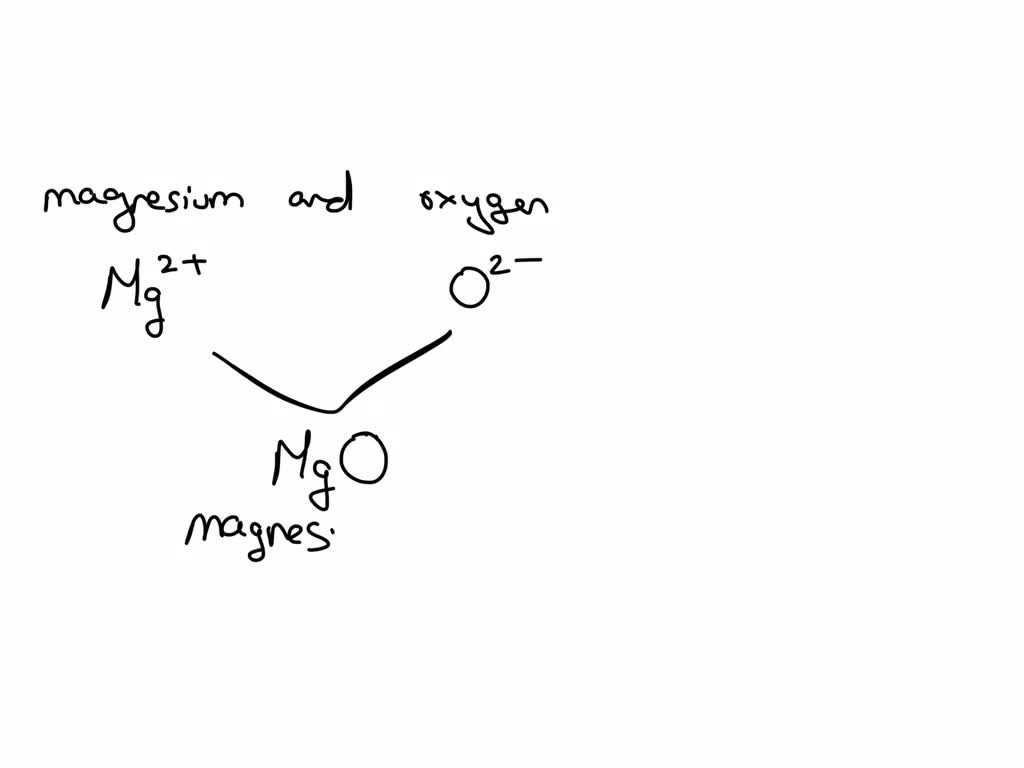
Magnesium Ion With O2- . The burning of magnesium metal reacts with oxygen found in. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. A magnesium atom will lose 2 electrons to form a stable 2 + ion. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Nitrogen’s position in the periodic table (group 15) reveals. Oxygen is in group 6 of the periodic table. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen.
from www.numerade.com
Nitrogen’s position in the periodic table (group 15) reveals. The burning of magnesium metal reacts with oxygen found in. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Oxygen is in group 6 of the periodic table. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. A magnesium atom will lose 2 electrons to form a stable 2 + ion.
SOLVED What happens when the compound Mgo is formed? (5 points) Oxygen
Magnesium Ion With O2- Oxygen is in group 6 of the periodic table. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Oxygen is in group 6 of the periodic table. Nitrogen’s position in the periodic table (group 15) reveals. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. A magnesium atom will lose 2 electrons to form a stable 2 + ion. The burning of magnesium metal reacts with oxygen found in.
From www.tessshebaylo.com
Chemical Equation For Synthesis Of Magnesium Oxide From And Oxygen Magnesium Ion With O2- A magnesium atom will lose 2 electrons to form a stable 2 + ion. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Magnesium oxide, also known. Magnesium Ion With O2-.
From www.slideserve.com
PPT Recap Atomic Structure PowerPoint Presentation, free download Magnesium Ion With O2- Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Nitrogen’s position in the periodic table (group 15) reveals. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Oxygen is. Magnesium Ion With O2-.
From kdi-ppi.com
Understanding the Magnesium Oxide Dot and Cross Diagram An Essential Guide Magnesium Ion With O2- Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −.. Magnesium Ion With O2-.
From www.youtube.com
Lewis Structure of Magnesium Oxide (MgO) YouTube Magnesium Ion With O2- The burning of magnesium metal reacts with oxygen found in. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Nitrogen’s position in the periodic table (group 15) reveals. Oxygen. Magnesium Ion With O2-.
From mungfali.com
Magnesium Comparison Chart Magnesium Ion With O2- When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Oxygen is in group 6 of the periodic table. The symbol for the ion is mg 2 +, and it is called a magnesium ion. The burning. Magnesium Ion With O2-.
From www.chemistryworld.com
Magnesium oxide Podcast Chemistry World Magnesium Ion With O2- Oxygen is in group 6 of the periodic table. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Nitrogen’s position in the periodic table (group 15) reveals. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen.. Magnesium Ion With O2-.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Magnesium General Wiring Diagram Magnesium Ion With O2- When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. A magnesium atom will lose 2 electrons to form a stable 2 + ion. The burning. Magnesium Ion With O2-.
From www.youtube.com
How to find the Oxidation Number for the Mg2+ ion. (Magnesium ion Magnesium Ion With O2- Oxygen is in group 6 of the periodic table. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. The burning of magnesium metal reacts with oxygen found. Magnesium Ion With O2-.
From www.youtube.com
Reaction of Oxygen and Magnesium YouTube Magnesium Ion With O2- When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. A magnesium atom will lose 2 electrons to form a stable 2 + ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The burning. Magnesium Ion With O2-.
From www.numerade.com
SOLVED Write the formula for an ionic compound formed from O2 with Magnesium Ion With O2- The burning of magnesium metal reacts with oxygen found in. Oxygen is in group 6 of the periodic table. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. When magnesium reacts. Magnesium Ion With O2-.
From www.numerade.com
SOLVED Draw the electron models for the magnesium ion (Mg2+) and the Magnesium Ion With O2- A magnesium atom will lose 2 electrons to form a stable 2 + ion. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Oxygen is in group 6 of the periodic table. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. The burning. Magnesium Ion With O2-.
From brainly.in
(i) Write the electron dot structures for sodium, oxygen and magnesium Magnesium Ion With O2- Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. A. Magnesium Ion With O2-.
From www.numerade.com
SOLVED What happens when the compound Mgo is formed? (5 points) Oxygen Magnesium Ion With O2- Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. When an ionic compound is formed from magnesium and oxygen, the. Magnesium Ion With O2-.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413898 Magnesium Ion With O2- Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. The. Magnesium Ion With O2-.
From www.slideshare.net
Bonding Basics Magnesium Ion With O2- Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. The burning of magnesium metal reacts with oxygen found in. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. The. Magnesium Ion With O2-.
From brainly.in
Formation of magnesium oxide from magnesium and oxygen Brainly.in Magnesium Ion With O2- Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen. Magnesium Ion With O2-.
From www.slideshare.net
Bonding Basics Magnesium Ion With O2- The burning of magnesium metal reacts with oxygen found in. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Nitrogen’s position in the periodic table (group 15) reveals. A magnesium atom will lose 2 electrons to form a stable 2 + ion. When an ionic compound is formed from magnesium and oxygen, the. Magnesium Ion With O2-.
From www.numerade.com
SOLVEDIf oxygen molecules, O2, were to react with magnesium atoms, how Magnesium Ion With O2- A magnesium atom will lose 2 electrons to form a stable 2 + ion. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Magnesium has a charge. Magnesium Ion With O2-.
From www.numerade.com
SOLVED Study the reaction equation. 2??+?2→2???2Mg+O2→2MgO If Magnesium Ion With O2- When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a. Magnesium Ion With O2-.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Ion With O2- The symbol for the ion is mg 2 +, and it is called a magnesium ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give. Magnesium Ion With O2-.
From www.youtube.com
Reaction of magnesium with oxygen gas 2Mg + O2 →2MgO Reaction of Magnesium Ion With O2- Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Oxygen is in group 6 of the periodic table. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. Nitrogen’s position in the periodic table. Magnesium Ion With O2-.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot Magnesium Ion With O2- When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Nitrogen’s position in the periodic table (group 15) reveals. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Oxygen is in. Magnesium Ion With O2-.
From www.numerade.com
SOLVEDBelow is pictured the reaction between an atom of magnesium and Magnesium Ion With O2- A magnesium atom will lose 2 electrons to form a stable 2 + ion. Nitrogen’s position in the periodic table (group 15) reveals. Oxygen is in group 6 of the periodic table. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. Magnesium has a charge of 2 +, while oxygen. Magnesium Ion With O2-.
From www.sciencephoto.com
Magnesium Oxide Stock Image A710/0103 Science Photo Library Magnesium Ion With O2- The symbol for the ion is mg 2 +, and it is called a magnesium ion. Oxygen is in group 6 of the periodic table. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Nitrogen’s position in the periodic table (group 15) reveals. Magnesium. Magnesium Ion With O2-.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Ion With O2- Oxygen is in group 6 of the periodic table. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Magnesium has. Magnesium Ion With O2-.
From www.slideserve.com
PPT BONDING PowerPoint Presentation, free download ID5456988 Magnesium Ion With O2- Oxygen is in group 6 of the periodic table. Nitrogen’s position in the periodic table (group 15) reveals. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. When an ionic compound. Magnesium Ion With O2-.
From treatybottle13.pythonanywhere.com
Fun Word Equation For Magnesium And Oxygen Mlt Dimensions Table Magnesium Ion With O2- When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The burning of magnesium metal reacts with oxygen found in. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. The symbol for the ion is. Magnesium Ion With O2-.
From www.showme.com
Magnesium + Oxygen Ionic Bonding Science, Chemistry ShowMe Magnesium Ion With O2- When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The symbol for the ion is mg 2 +, and it is called a magnesium ion. When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen.. Magnesium Ion With O2-.
From www.showme.com
Formula for Magnesium oxide Science, Chemistry ShowMe Magnesium Ion With O2- A magnesium atom will lose 2 electrons to form a stable 2 + ion. Nitrogen’s position in the periodic table (group 15) reveals. Oxygen is in group 6 of the periodic table. The burning of magnesium metal reacts with oxygen found in. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to. Magnesium Ion With O2-.
From diagramlibraryverb.z13.web.core.windows.net
Dot And Cross Diagram For Magnesium Oxide Magnesium Ion With O2- A magnesium atom will lose 2 electrons to form a stable 2 + ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Nitrogen’s position in the periodic table (group 15) reveals. The symbol for the ion is mg 2 +, and it is. Magnesium Ion With O2-.
From mammothmemory.net
Valency is the number of bonds an atom can make with others Magnesium Ion With O2- When magnesium reacts with oxygen, the magnesium atoms donate electrons to o 2 molecules and thereby reduce the oxygen. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Nitrogen’s position in the periodic table (group 15) reveals. When an ionic. Magnesium Ion With O2-.
From brainly.in
Draw the atomic structure of oxygen ion (O 2) Brainly.in Magnesium Ion With O2- When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Nitrogen’s position in the periodic table (group 15) reveals.. Magnesium Ion With O2-.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion With O2- Magnesium is a silvery metal that is quite active, reacting slowly with boiling (but not cold) water to give hydrogen and the rather insoluble magnesium hydroxide. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium. Magnesium Ion With O2-.
From childhealthpolicy.vumc.org
⛔ Chemical reaction of magnesium and oxygen. What is the chemical Magnesium Ion With O2- A magnesium atom will lose 2 electrons to form a stable 2 + ion. The symbol for the ion is mg 2 +, and it is called a magnesium ion. Nitrogen’s position in the periodic table (group 15) reveals. Oxygen is in group 6 of the periodic table. When an ionic compound is formed from magnesium and oxygen, the magnesium. Magnesium Ion With O2-.
From articleeducation.x.fc2.com
Explain how magnesium and oxygen atoms react to form magnesium oxide Magnesium Ion With O2- When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Oxygen is in group 6 of the periodic table. Magnesium has a charge of 2 +, while oxygen has a charge. Magnesium Ion With O2-.