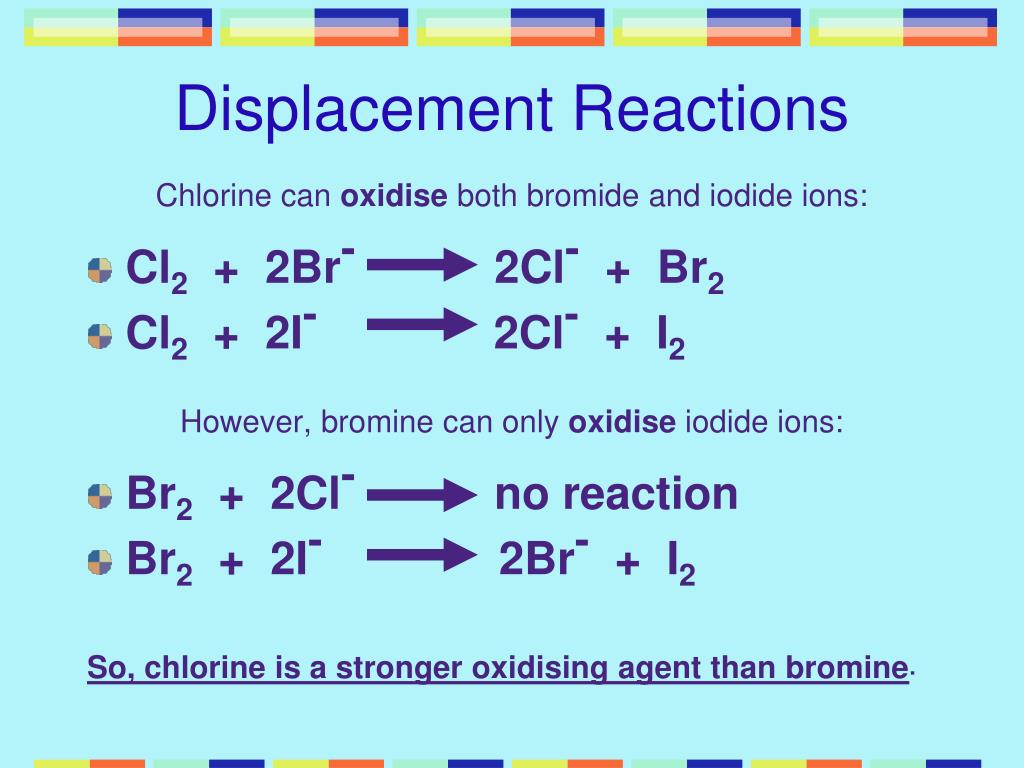
Chlorine And Bromine Are Both Oxidising Agents . chlorine also reacts with water, but only in the presence of sunlight. Bromine is weaker, and iodine has only mild oxidizing power. chlorine and bromine are both oxidising agents. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. this page examines the trend in oxidizing ability of the group 17 elements (the halogens): (a) (b) define an oxidising agent in terms of electrons. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. Here chlorine and bromine are strong.
from www.slideserve.com
Bromine is weaker, and iodine has only mild oxidizing power. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: this page examines the trend in oxidizing ability of the group 17 elements (the halogens): chlorine and bromine are both oxidising agents. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. Here chlorine and bromine are strong. (a) (b) define an oxidising agent in terms of electrons. chlorine also reacts with water, but only in the presence of sunlight.
PPT Halogens PowerPoint Presentation, free download ID2110165
Chlorine And Bromine Are Both Oxidising Agents Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. this page examines the trend in oxidizing ability of the group 17 elements (the halogens): for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Bromine is weaker, and iodine has only mild oxidizing power. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. chlorine also reacts with water, but only in the presence of sunlight. chlorine and bromine are both oxidising agents. (a) (b) define an oxidising agent in terms of electrons. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Here chlorine and bromine are strong.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Chlorine And Bromine Are Both Oxidising Agents for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. chlorine and bromine are both oxidising agents. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. Bromine is weaker,. Chlorine And Bromine Are Both Oxidising Agents.
From www.poolsuppliessuperstore.com
Bromine vs. Chlorine Everything You Need to Know Pool Supplies Chlorine And Bromine Are Both Oxidising Agents chlorine and bromine are both oxidising agents. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. (a) (b) define an oxidising agent in terms of electrons. we can demonstrate. Chlorine And Bromine Are Both Oxidising Agents.
From www.numerade.com
SOLVEDAlthough chlorine is a stronger oxidizing agent than bromine Chlorine And Bromine Are Both Oxidising Agents this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Bromine. Chlorine And Bromine Are Both Oxidising Agents.
From www.slideserve.com
PPT AQA AS Chemistry Revision PowerPoint Presentation, free download Chlorine And Bromine Are Both Oxidising Agents Here chlorine and bromine are strong. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. chlorine also reacts with water, but only in the presence of sunlight. (a) (b) define an oxidising agent in terms of electrons. Bromine is weaker, and iodine has only mild oxidizing power.. Chlorine And Bromine Are Both Oxidising Agents.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Chlorine And Bromine Are Both Oxidising Agents we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. (a) (b) define an oxidising agent in terms of electrons. Bromine is weaker, and iodine has only mild oxidizing power. Here chlorine and bromine are strong. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table.. Chlorine And Bromine Are Both Oxidising Agents.
From www.spaworld.com.au
What's better for a spa Chlorine or Bromine? Chlorine And Bromine Are Both Oxidising Agents we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. Bromine is weaker, and iodine has only mild oxidizing power. Here chlorine and bromine are strong. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. for example, chlorine can oxidise the bromide ions (in,. Chlorine And Bromine Are Both Oxidising Agents.
From www.prettypracticalhome.com
Chlorine Versus Bromine Things to know about them Chlorine And Bromine Are Both Oxidising Agents in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. (a) (b) define an oxidising agent in terms of electrons. chlorine also reacts with water, but only in the presence of sunlight. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: chlorine. Chlorine And Bromine Are Both Oxidising Agents.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Chlorine And Bromine Are Both Oxidising Agents (a) (b) define an oxidising agent in terms of electrons. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. chlorine also reacts with water, but only in the presence of. Chlorine And Bromine Are Both Oxidising Agents.
From sciencestruck.com
Bromine Vs. Chlorine Science Struck Chlorine And Bromine Are Both Oxidising Agents Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. Here chlorine and bromine are strong. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. Bromine is weaker, and iodine has only mild oxidizing power. chlorine also reacts with water, but only in. Chlorine And Bromine Are Both Oxidising Agents.
From pediaa.com
Difference Between Bromine and Chlorine Chlorine And Bromine Are Both Oxidising Agents this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. chlorine also reacts with water, but only in the presence of sunlight. (a) (b) define an oxidising agent in terms of electrons. chlorine and bromine are both oxidising agents. we can demonstrate the oxidising ability of. Chlorine And Bromine Are Both Oxidising Agents.
From www.numerade.com
SOLVED Consider the reaction of bromide and chlorine Balance the Chlorine And Bromine Are Both Oxidising Agents for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: (a) (b) define an oxidising agent in terms of electrons. this page examines the trend in oxidizing ability of the group 17 elements (the halogens): Bromine is weaker, and iodine has only mild oxidizing power. we can demonstrate the oxidising ability. Chlorine And Bromine Are Both Oxidising Agents.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Chlorine And Bromine Are Both Oxidising Agents for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: this page examines the trend in oxidizing ability of the group 17 elements (the halogens): we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. this article contains comparison of key. Chlorine And Bromine Are Both Oxidising Agents.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Are Both Oxidising Agents we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. Bromine is weaker, and iodine has only mild oxidizing power. Bromine and chlorine are both halogens, belonging to group. Chlorine And Bromine Are Both Oxidising Agents.
From www.slideserve.com
PPT What is Electrochemistry? PowerPoint Presentation, free download Chlorine And Bromine Are Both Oxidising Agents for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: chlorine and bromine are both oxidising agents. (a) (b) define an oxidising agent in terms of electrons. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. chlorine also reacts with water, but. Chlorine And Bromine Are Both Oxidising Agents.
From www.qcchlorine.com
The Difference Between Chlorine and Bromine in Swimming Pool Chlorine And Bromine Are Both Oxidising Agents for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: (a) (b) define an oxidising agent in terms of electrons. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. Bromine is weaker, and iodine has only mild oxidizing power. chlorine also. Chlorine And Bromine Are Both Oxidising Agents.
From slideplayer.com
Do Now How many possible reactions are there when Group 1 and 2 Chlorine And Bromine Are Both Oxidising Agents Here chlorine and bromine are strong. chlorine also reacts with water, but only in the presence of sunlight. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. we. Chlorine And Bromine Are Both Oxidising Agents.
From www.quora.com
Does Chlorine and Bromine exhibit +4 and +6 oxidation state in oxides Chlorine And Bromine Are Both Oxidising Agents we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. (a) (b) define an oxidising agent in terms of electrons. Here chlorine and bromine are strong. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Bromine and chlorine are both halogens, belonging. Chlorine And Bromine Are Both Oxidising Agents.
From www.slideserve.com
PPT Q9 What are the chlorine and bromine reactions that destroy Chlorine And Bromine Are Both Oxidising Agents Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. chlorine also reacts with water, but only in the presence of sunlight. chlorine and bromine are both oxidising agents. this page examines the trend in oxidizing ability of the group 17 elements (the halogens): (a) (b) define an oxidising agent in terms of. Chlorine And Bromine Are Both Oxidising Agents.
From www.browninghottubs.com
Hot Tub Sanitizers Chlorine vs. Bromine — Browning Hot Tubs Chlorine And Bromine Are Both Oxidising Agents Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. this page examines the trend in oxidizing ability of the group 17 elements (the halogens): for example, chlorine can oxidise the bromide ions (in,. Chlorine And Bromine Are Both Oxidising Agents.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Chlorine And Bromine Are Both Oxidising Agents in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Here chlorine and bromine are strong. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. chlorine also reacts with water, but only in the presence of sunlight. this page examines the trend in oxidizing ability. Chlorine And Bromine Are Both Oxidising Agents.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Are Both Oxidising Agents chlorine also reacts with water, but only in the presence of sunlight. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Bromine is weaker, and iodine has only mild oxidizing power. Here chlorine. Chlorine And Bromine Are Both Oxidising Agents.
From chem.libretexts.org
10.6.2. Strong Oxidizing Agents Chemistry LibreTexts Chlorine And Bromine Are Both Oxidising Agents (a) (b) define an oxidising agent in terms of electrons. Bromine is weaker, and iodine has only mild oxidizing power. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: chlorine and bromine are. Chlorine And Bromine Are Both Oxidising Agents.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5566024 Chlorine And Bromine Are Both Oxidising Agents Bromine is weaker, and iodine has only mild oxidizing power. this page examines the trend in oxidizing ability of the group 17 elements (the halogens): chlorine also reacts with water, but only in the presence of sunlight. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. for example, chlorine can oxidise the. Chlorine And Bromine Are Both Oxidising Agents.
From www.studocu.com
3.1.7 Oxidationreductionredox eqns Chlorine and bromine are both Chlorine And Bromine Are Both Oxidising Agents Bromine is weaker, and iodine has only mild oxidizing power. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Here chlorine and bromine are strong. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: (a) (b) define an oxidising agent in terms of. Chlorine And Bromine Are Both Oxidising Agents.
From slideplayer.com
3.16 Oxidising and Reducing Agents ppt download Chlorine And Bromine Are Both Oxidising Agents we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. chlorine and bromine are both oxidising agents. (a) (b) define an oxidising agent in terms of electrons. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Bromine is weaker, and iodine. Chlorine And Bromine Are Both Oxidising Agents.
From spasearch.org
Chlorine vs. Bromine Which is Best for Your Home Spa? Chlorine And Bromine Are Both Oxidising Agents chlorine and bromine are both oxidising agents. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Bromine is weaker, and iodine has only mild oxidizing power. Here chlorine and bromine are strong. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron. Chlorine And Bromine Are Both Oxidising Agents.
From www.chemistrystudent.com
Group 7 Halogens Oxidising Power (ALevel) ChemistryStudent Chlorine And Bromine Are Both Oxidising Agents (a) (b) define an oxidising agent in terms of electrons. Bromine is weaker, and iodine has only mild oxidizing power. Here chlorine and bromine are strong. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two. Chlorine And Bromine Are Both Oxidising Agents.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Chlorine And Bromine Are Both Oxidising Agents Bromine is weaker, and iodine has only mild oxidizing power. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: chlorine also reacts with water, but only in the presence of. Chlorine And Bromine Are Both Oxidising Agents.
From www.alvimcleantech.com
Redox potential Chlorine And Bromine Are Both Oxidising Agents we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. Bromine. Chlorine And Bromine Are Both Oxidising Agents.
From www.slideshare.net
Oxidation & reduction Chlorine And Bromine Are Both Oxidising Agents in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: Bromine is weaker, and iodine has only mild oxidizing power. . Chlorine And Bromine Are Both Oxidising Agents.
From differencebetweenz.com
Difference between Bromine and Chlorine Difference Betweenz Chlorine And Bromine Are Both Oxidising Agents Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. Here chlorine and bromine are strong. this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. (a) (b) define an oxidising agent in terms of electrons. in the displacement reactions chlorine displaces both bromine. Chlorine And Bromine Are Both Oxidising Agents.
From slideplayer.com
Topic 3 Periodicity. ppt download Chlorine And Bromine Are Both Oxidising Agents for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: this article contains comparison of key thermal and atomic properties of chlorine and bromine, two comparable chemical elements from the. Here chlorine and bromine are strong. this page examines the trend in oxidizing ability of the group 17 elements (the halogens):. Chlorine And Bromine Are Both Oxidising Agents.
From slideplayer.com
PART 2 The Periodic Table and Chemical Properties of Groups 1 & 7 Chlorine And Bromine Are Both Oxidising Agents Here chlorine and bromine are strong. we can demonstrate the oxidising ability of the halogens by reacting them with a solution of iron (ii) chloride. Bromine is weaker, and iodine has only mild oxidizing power. (a) (b) define an oxidising agent in terms of electrons. chlorine and bromine are both oxidising agents. Bromine and chlorine are both halogens,. Chlorine And Bromine Are Both Oxidising Agents.
From www.expii.com
Oxidizing and Reducing Agents — Definition & Examples Expii Chlorine And Bromine Are Both Oxidising Agents in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Bromine and chlorine are both halogens, belonging to group 17 of the periodic table. chlorine also reacts with water, but only in the presence of sunlight. Bromine is weaker, and iodine has only mild oxidizing power. this article contains comparison. Chlorine And Bromine Are Both Oxidising Agents.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Chlorine And Bromine Are Both Oxidising Agents for example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. chlorine and bromine are both oxidising agents. Bromine is weaker, and iodine has only mild oxidizing power. we can demonstrate the oxidising ability. Chlorine And Bromine Are Both Oxidising Agents.