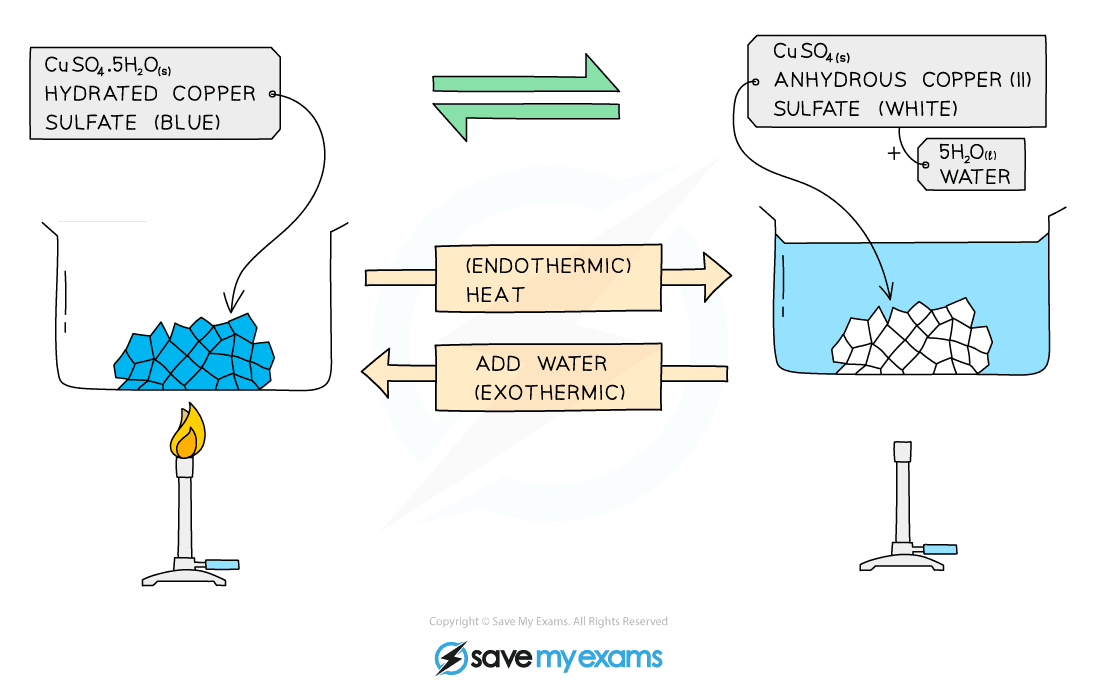
Copper Ii Sulfate Hydrate Empirical Formula . This form is characterized by its. the formula of a hydrate is represented in a special manner. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. Here is the formula for hydrated copper. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. It forms hydrate s cuso4·nh2o,. this video discusses how to analyze data from an experiment. This is a class experiment suitable for students who. water of crystallisation is water that is chemically bonded into a crystal structure. The hydrate of copper (ii) sulfate in this experiment. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4.
from classnotes.gidemy.com
Here is the formula for hydrated copper. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. This form is characterized by its. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. this video discusses how to analyze data from an experiment. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. The hydrate of copper (ii) sulfate in this experiment. It forms hydrate s cuso4·nh2o,. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. water of crystallisation is water that is chemically bonded into a crystal structure.
Reversible Reactions Gidemy Class Notes
Copper Ii Sulfate Hydrate Empirical Formula Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. Here is the formula for hydrated copper. the formula of a hydrate is represented in a special manner. It forms hydrate s cuso4·nh2o,. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. This form is characterized by its. this video discusses how to analyze data from an experiment. The hydrate of copper (ii) sulfate in this experiment. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. water of crystallisation is water that is chemically bonded into a crystal structure. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. This is a class experiment suitable for students who.
From www.vrogue.co
How To Write The Formula For Copper Ii Sulfate Youtub vrogue.co Copper Ii Sulfate Hydrate Empirical Formula water of crystallisation is water that is chemically bonded into a crystal structure. This is a class experiment suitable for students who. This form is characterized by its. this video discusses how to analyze data from an experiment. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation.. Copper Ii Sulfate Hydrate Empirical Formula.
From www.studypool.com
SOLUTION Empirical formula of hydrated copper ii sulfate the accuracy Copper Ii Sulfate Hydrate Empirical Formula This is a class experiment suitable for students who. water of crystallisation is water that is chemically bonded into a crystal structure. Here is the formula for hydrated copper. the formula of a hydrate is represented in a special manner. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. the most common form. Copper Ii Sulfate Hydrate Empirical Formula.
From www.studypool.com
SOLUTION Empirical formula of hydrated copper ii sulfate the accuracy Copper Ii Sulfate Hydrate Empirical Formula water of crystallisation is water that is chemically bonded into a crystal structure. this video discusses how to analyze data from an experiment. the formula of a hydrate is represented in a special manner. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses. Copper Ii Sulfate Hydrate Empirical Formula.
From studylib.net
Determination of the Formula of Copper II Sulfate Copper Ii Sulfate Hydrate Empirical Formula water of crystallisation is water that is chemically bonded into a crystal structure. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. this video discusses how to analyze data from an experiment. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. Here is. Copper Ii Sulfate Hydrate Empirical Formula.
From informacionpublica.svet.gob.gt
How Do You Know When Copper(II) Sulfate Hydrated Water Copper Ii Sulfate Hydrate Empirical Formula This form is characterized by its. This is a class experiment suitable for students who. It forms hydrate s cuso4·nh2o,. the formula of a hydrate is represented in a special manner. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. in this experiment, a known mass of. Copper Ii Sulfate Hydrate Empirical Formula.
From www.youtube.com
Finding the Empirical Formula of Hydrated Copper (II) Sulfate (Updated Copper Ii Sulfate Hydrate Empirical Formula in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. Here is the formula for hydrated copper. this video discusses how to analyze data from an experiment. Copper (ii). Copper Ii Sulfate Hydrate Empirical Formula.
From www.sciencephoto.com
Hydrated copper (II) sulphate crystals Stock Image C004/7690 Copper Ii Sulfate Hydrate Empirical Formula in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. This is a class experiment suitable for students who. water of crystallisation is water that is chemically bonded into a crystal structure. This form is characterized by its. this video discusses how to analyze data from an experiment.. Copper Ii Sulfate Hydrate Empirical Formula.
From studylib.net
Determining the Chemical Formula of Copper (II) Sulfate Hydrate Copper Ii Sulfate Hydrate Empirical Formula This form is characterized by its. the formula of a hydrate is represented in a special manner. Here is the formula for hydrated copper. This is a class experiment suitable for students who. It forms hydrate s cuso4·nh2o,. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air,. Copper Ii Sulfate Hydrate Empirical Formula.
From slideplayer.com
Chapter Thirteen CHEMICAL EQUILIBRIUM. ppt download Copper Ii Sulfate Hydrate Empirical Formula this video discusses how to analyze data from an experiment. the formula of a hydrate is represented in a special manner. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. This form is characterized by its. This is a class experiment suitable for students who. when. Copper Ii Sulfate Hydrate Empirical Formula.
From www.studocu.com
Lab report Empirical Formula of Hydrated Copper (II) Sulfate The Copper Ii Sulfate Hydrate Empirical Formula The hydrate of copper (ii) sulfate in this experiment. the formula of a hydrate is represented in a special manner. This form is characterized by its. water of crystallisation is water that is chemically bonded into a crystal structure. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of. Copper Ii Sulfate Hydrate Empirical Formula.
From www.numerade.com
Chemistry 110 Laboratory Water Content Empirical Formulas for Hydrated Copper Ii Sulfate Hydrate Empirical Formula This is a class experiment suitable for students who. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. Here is the formula for hydrated. Copper Ii Sulfate Hydrate Empirical Formula.
From www.youtube.com
Empirical formula copper II sulfate YouTube Copper Ii Sulfate Hydrate Empirical Formula It forms hydrate s cuso4·nh2o,. the formula of a hydrate is represented in a special manner. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. in this experiment, a known mass of hydrated copper. Copper Ii Sulfate Hydrate Empirical Formula.
From www.slideserve.com
PPT Water of crystallisation PowerPoint Presentation, free download Copper Ii Sulfate Hydrate Empirical Formula This is a class experiment suitable for students who. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. It forms hydrate s cuso4·nh2o,. the formula of a hydrate is represented in a special manner. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules. Copper Ii Sulfate Hydrate Empirical Formula.
From cupsoguepictures.com
😎 What is the empirical formula of copper sulfate hydrate. Formula of a Copper Ii Sulfate Hydrate Empirical Formula this video discusses how to analyze data from an experiment. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. It forms hydrate s. Copper Ii Sulfate Hydrate Empirical Formula.
From www.studocu.com
Experiment 4 Empirical formula of hydrated copper (II) sulfate Copper Ii Sulfate Hydrate Empirical Formula when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. It forms hydrate s cuso4·nh2o,. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. the most common form of copper sulfate is its. Copper Ii Sulfate Hydrate Empirical Formula.
From www.numerade.com
SOLVEDA hydrate of copper(II) sulfate, when heated, goes through the Copper Ii Sulfate Hydrate Empirical Formula in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. It forms hydrate s cuso4·nh2o,. The hydrate of copper (ii) sulfate in this experiment. This. Copper Ii Sulfate Hydrate Empirical Formula.
From www.youtube.com
How to Write the Formula for Copper (II) sulfate YouTube Copper Ii Sulfate Hydrate Empirical Formula water of crystallisation is water that is chemically bonded into a crystal structure. the formula of a hydrate is represented in a special manner. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. Here is the formula for hydrated copper. It forms hydrate s cuso4·nh2o,. The hydrate. Copper Ii Sulfate Hydrate Empirical Formula.
From es.scribd.com
Determination of the Empirical and Molecular Formulas of a Hydrated Copper Ii Sulfate Hydrate Empirical Formula the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. water of crystallisation is water that is chemically bonded into a crystal structure. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. . Copper Ii Sulfate Hydrate Empirical Formula.
From www.studocu.com
laboratory experiment four chemistry lab Empirical Formula of Copper Ii Sulfate Hydrate Empirical Formula water of crystallisation is water that is chemically bonded into a crystal structure. This form is characterized by its. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. The hydrate of copper (ii) sulfate in this experiment. in this experiment, a known. Copper Ii Sulfate Hydrate Empirical Formula.
From www.numerade.com
SOLVED You heat sample of copper(II) sulfate hydrate to drive off the Copper Ii Sulfate Hydrate Empirical Formula this video discusses how to analyze data from an experiment. Here is the formula for hydrated copper. It forms hydrate s cuso4·nh2o,. This is a class experiment suitable for students who. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to. Copper Ii Sulfate Hydrate Empirical Formula.
From www.chegg.com
Solved Empirical Formula of a Hydrate Data Experiment 1 Copper Ii Sulfate Hydrate Empirical Formula the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. this video discusses how to analyze data from an experiment. This is a class experiment suitable for students who. water of crystallisation is water that is chemically bonded into a crystal structure. Here is the formula for hydrated. Copper Ii Sulfate Hydrate Empirical Formula.
From www.chegg.com
Solved Table 2. Empirical Formula of a Copper (II) Sulfate Copper Ii Sulfate Hydrate Empirical Formula This is a class experiment suitable for students who. water of crystallisation is water that is chemically bonded into a crystal structure. this video discusses how to analyze data from an experiment. The hydrate of copper (ii) sulfate in this experiment. Here is the formula for hydrated copper. the formula of a hydrate is represented in a. Copper Ii Sulfate Hydrate Empirical Formula.
From childhealthpolicy.vumc.org
🌷 Hydrated copper sulfate equation. Finding the formula of hydrated Copper Ii Sulfate Hydrate Empirical Formula Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. the formula of a hydrate is represented in a special manner. This is a class experiment suitable for students who. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. this video discusses how to. Copper Ii Sulfate Hydrate Empirical Formula.
From dxowarewl.blob.core.windows.net
Copper Ii Sulfate Solubility In Water G/L at Marguerite Firestone blog Copper Ii Sulfate Hydrate Empirical Formula when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. water of crystallisation is water that is chemically bonded into a crystal structure. this video discusses how to analyze data from an experiment. the most common form of copper sulfate is its. Copper Ii Sulfate Hydrate Empirical Formula.
From edu.rsc.org
Finding the formula of hydrated copper(II) sulfate Experiment RSC Copper Ii Sulfate Hydrate Empirical Formula This form is characterized by its. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. water of crystallisation is water that is chemically bonded into a crystal structure. The hydrate of copper (ii) sulfate in this experiment. Here is the formula for hydrated copper. It forms hydrate s. Copper Ii Sulfate Hydrate Empirical Formula.
From www.slideserve.com
PPT Percent Composition With Hydrates & Empirical Formulas PowerPoint Copper Ii Sulfate Hydrate Empirical Formula water of crystallisation is water that is chemically bonded into a crystal structure. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. It forms hydrate s cuso4·nh2o,. this video discusses how to analyze data from an experiment. This form is characterized by its. when copper (ii). Copper Ii Sulfate Hydrate Empirical Formula.
From edu.rsc.org
Finding the formula of hydrated copper(II) sulfate Experiment RSC Copper Ii Sulfate Hydrate Empirical Formula It forms hydrate s cuso4·nh2o,. This is a class experiment suitable for students who. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. water of crystallisation is water that is chemically bonded into a crystal structure. the formula of a hydrate is represented in a special manner.. Copper Ii Sulfate Hydrate Empirical Formula.
From studylib.net
To find the formula of hydrated copper(II) sulfate Copper Ii Sulfate Hydrate Empirical Formula It forms hydrate s cuso4·nh2o,. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it. Copper Ii Sulfate Hydrate Empirical Formula.
From www.fishersci.co.uk
Copper(II) sulfate hydrate, Puratronic , 99.999 (metals basis), Thermo Copper Ii Sulfate Hydrate Empirical Formula This form is characterized by its. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. The hydrate of copper (ii) sulfate in this experiment. the formula of a hydrate is represented in a special manner. the most common form of copper sulfate is its pentahydrate, given by. Copper Ii Sulfate Hydrate Empirical Formula.
From www.tessshebaylo.com
Write A Balanced Chemical Equation For The Of Copper Ii Copper Ii Sulfate Hydrate Empirical Formula It forms hydrate s cuso4·nh2o,. This form is characterized by its. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. This is a class experiment suitable for students who. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o.. Copper Ii Sulfate Hydrate Empirical Formula.
From www.scribd.com
Determine The Empirical Formula of Hydrated Copper Sulfate Background Copper Ii Sulfate Hydrate Empirical Formula in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. This is a class experiment suitable for students who. when copper (ii) sulfate hydrate, a blue crystalline solid containing. Copper Ii Sulfate Hydrate Empirical Formula.
From chem11lucas.wordpress.com
Hydrates Chemistry Copper Ii Sulfate Hydrate Empirical Formula water of crystallisation is water that is chemically bonded into a crystal structure. Here is the formula for hydrated copper. when copper (ii) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water. the formula of a hydrate is represented in a special manner. Copper (ii). Copper Ii Sulfate Hydrate Empirical Formula.
From www.sciencephoto.com
Hydrated copper (II) sulphate Stock Image C009/9431 Science Photo Copper Ii Sulfate Hydrate Empirical Formula this video discusses how to analyze data from an experiment. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. It forms hydrate s cuso4·nh2o,. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. This is a class experiment suitable for students who. This form. Copper Ii Sulfate Hydrate Empirical Formula.
From classnotes.gidemy.com
Reversible Reactions Gidemy Class Notes Copper Ii Sulfate Hydrate Empirical Formula This is a class experiment suitable for students who. This form is characterized by its. water of crystallisation is water that is chemically bonded into a crystal structure. Copper (ii) sulfate is an inorganic compound with the chemical formula cu so4. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h. Copper Ii Sulfate Hydrate Empirical Formula.
From dxojfqcbd.blob.core.windows.net
Copper Ii Oxide To Copper Ii Sulfate Equation at Gloria Schirmer blog Copper Ii Sulfate Hydrate Empirical Formula the formula of a hydrate is represented in a special manner. water of crystallisation is water that is chemically bonded into a crystal structure. the most common form of copper sulfate is its pentahydrate, given by the chemical formula cuso 4.5h 2 o. This form is characterized by its. Here is the formula for hydrated copper. . Copper Ii Sulfate Hydrate Empirical Formula.