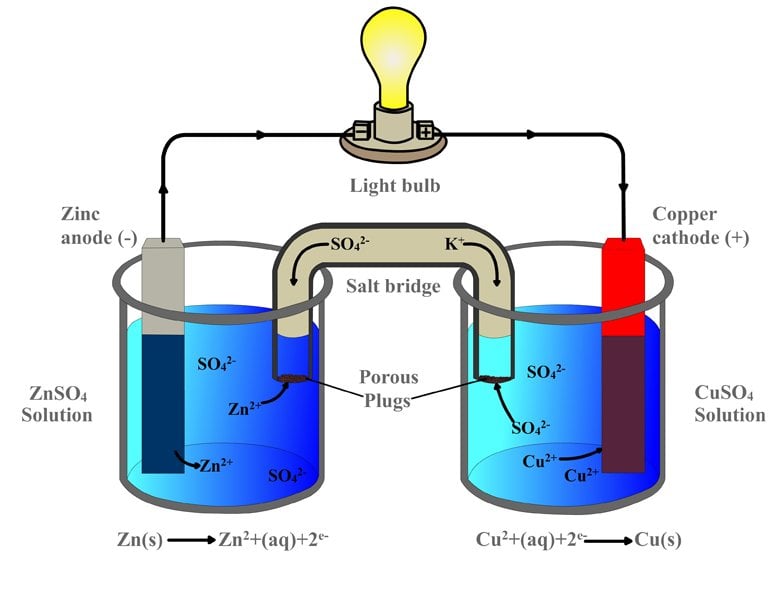
In A Galvanic Cell Reduction Occurs At Which Electrode . The anode has a negative potential with respect to. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In writing the equations, it is often. The positive electrode, on the other hand, will attract negative ions (anions). Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode.
from www.scienceabc.com
In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions).
Galvanic Cell Definition, Diagram And Working
In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. The anode has a negative potential with respect to. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 In A Galvanic Cell Reduction Occurs At Which Electrode The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions). Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic). In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID9200026 In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential with respect to. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.numerade.com
Galvanic cell based on the above reaction constructed according to the In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place. In A Galvanic Cell Reduction Occurs At Which Electrode.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.numerade.com
SOLVED In the galvanic cell, reduction occurs at the and in the In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From ruby-blogcampbell.blogspot.com
The Electrode Where Reduction Occurs Is Called the Anode In A Galvanic Cell Reduction Occurs At Which Electrode The positive electrode, on the other hand, will attract negative ions (anions). Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.numerade.com
SOLVEDQuestion 32 2 pts In a galvanic cell, reduction occurs at the In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive electrode, on the other hand, will attract. In A Galvanic Cell Reduction Occurs At Which Electrode.
From sites.google.com
Electrochemistry engineering chemistry In A Galvanic Cell Reduction Occurs At Which Electrode Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions). In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is. In A Galvanic Cell Reduction Occurs At Which Electrode.
From loeduhpag.blob.core.windows.net
Hydrogen Electrode Galvanic Cell at Justin Mclean blog In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.slideserve.com
PPT Galvanic cell PowerPoint Presentation, free download ID2330768 In A Galvanic Cell Reduction Occurs At Which Electrode Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential with respect to. In writing the equations, it is often. The positive electrode, on the other hand, will attract negative ions (anions). In any electrochemical cell (electrolytic or galvanic). In A Galvanic Cell Reduction Occurs At Which Electrode.
From courses.lumenlearning.com
Galvanic Cells General Chemistry In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. The positive electrode, on the other hand, will attract negative ions (anions). In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive electrode, on the other hand, will attract negative ions. In A Galvanic Cell Reduction Occurs At Which Electrode.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. The positive electrode, on the other hand, will attract negative ions (anions). Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential with respect to. In any electrochemical cell (electrolytic or galvanic). In A Galvanic Cell Reduction Occurs At Which Electrode.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.numerade.com
SOLVED Suppose the galvanic cell sketched below is powered by the In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In writing the equations, it is often. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.
From stock.adobe.com
A galvanic cell or voltaic cell, is an electrochemical cell in which an In A Galvanic Cell Reduction Occurs At Which Electrode The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.numerade.com
SOLVED Using the image of the galvanic cell below, what is the In A Galvanic Cell Reduction Occurs At Which Electrode The positive electrode, on the other hand, will attract negative ions (anions). Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.tes.com
Galvanic Cells, Anode, Cathode, Oxidation, Reduction, Grade 12 In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive electrode, on the other hand, will attract negative ions. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1951076 In A Galvanic Cell Reduction Occurs At Which Electrode Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. In writing the equations, it is often. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential with respect to. In writing the equations, it is often. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.
From mungfali.com
Line Notation Galvanic Cell In A Galvanic Cell Reduction Occurs At Which Electrode The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions). In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place. In A Galvanic Cell Reduction Occurs At Which Electrode.
From 2012books.lardbucket.org
Describing Electrochemical Cells In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.dreamstime.com
Simple Electrochemical or Galvanic Cell. the Daniell Cell. Stock Vector In A Galvanic Cell Reduction Occurs At Which Electrode The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In any electrochemical cell (electrolytic or galvanic). In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential. In A Galvanic Cell Reduction Occurs At Which Electrode.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts In A Galvanic Cell Reduction Occurs At Which Electrode Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In writing the equations, it is often. The anode has a negative potential with respect to. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.
From allysonkruwnelson.blogspot.com
Where Does Reduction Occur in an Electrochemical Cell AllysonkruwNelson In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). The anode has a negative potential with respect to. In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From kladwjlgf.blob.core.windows.net
Electrochemistry Galvanic Cells at Roberto Alvarado blog In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). The anode has a negative potential with respect to. In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.dynamicscience.com.au
chemistry galvanic cells In A Galvanic Cell Reduction Occurs At Which Electrode Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. In A Galvanic Cell Reduction Occurs At Which Electrode.
From courses.lumenlearning.com
Galvanic Cells General Chemistry In A Galvanic Cell Reduction Occurs At Which Electrode The anode has a negative potential with respect to. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. In any electrochemical cell (electrolytic or galvanic). In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.numerade.com
SOLVED Label each half cell in this diagram of a voltaic cell to In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called. In A Galvanic Cell Reduction Occurs At Which Electrode.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download In A Galvanic Cell Reduction Occurs At Which Electrode In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The anode has a negative potential with respect to. The positive electrode, on the other hand, will attract negative ions (anions). In any electrochemical cell (electrolytic or galvanic). In A Galvanic Cell Reduction Occurs At Which Electrode.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell In A Galvanic Cell Reduction Occurs At Which Electrode In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode has a negative potential with respect to. In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts In A Galvanic Cell Reduction Occurs At Which Electrode The anode has a negative potential with respect to. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In writing the equations, it is often. Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode. The positive. In A Galvanic Cell Reduction Occurs At Which Electrode.