Medical Device Packaging And Labeling . Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. The ce marking should be affixed to the device or its sterile packaging. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet.
from www.slideshare.net
•understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. The ce marking should be affixed to the device or its sterile packaging. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd).
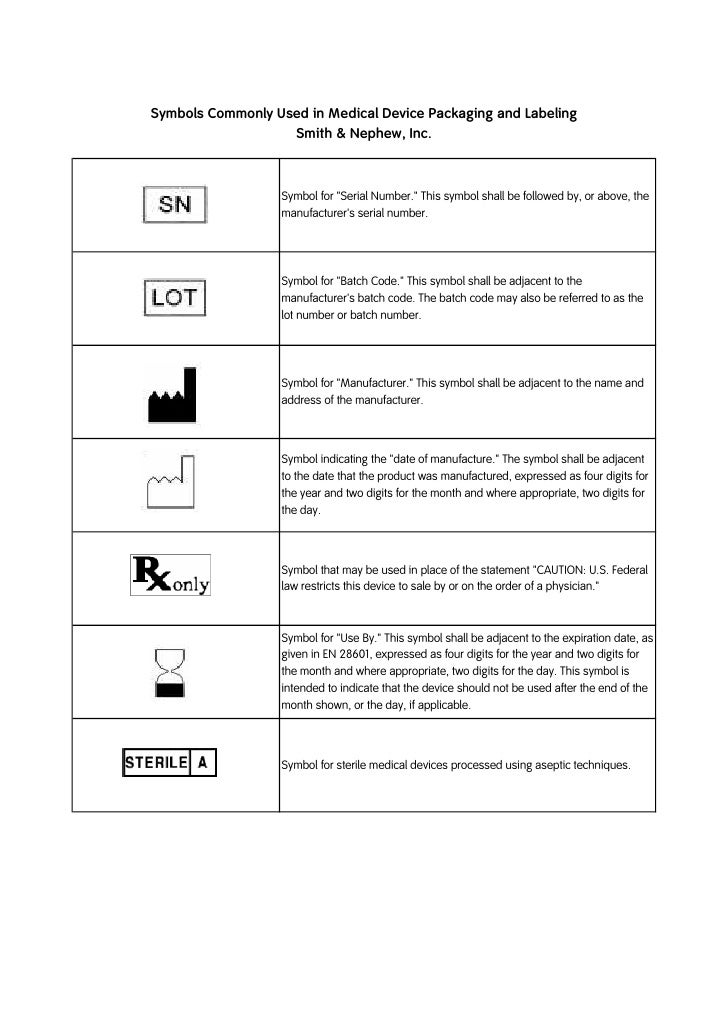
Symbols Commonly Used in Medical Device Packaging and Labeling
Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). The ce marking should be affixed to the device or its sterile packaging. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd).
From clin-r.com
Labels for Medical Devices Clin R Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling plays. Medical Device Packaging And Labeling.
From exyzsultp.blob.core.windows.net
Fda Guidance Medical Device Patient Labeling at Jana Flores blog Medical Device Packaging And Labeling Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. The ce marking should be affixed to the device or its sterile packaging. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr).. Medical Device Packaging And Labeling.
From www.slideshare.net
Symbols Commonly Used in Medical Device Packaging and Labeling Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Labeling and packaging is key. Medical Device Packaging And Labeling.
From tataelxsi.com
Tata Elxsi Medical Device Packaging & Labeling Services Medical Device Packaging And Labeling Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. •understand the requirements for medical device labeling •review some key labeling provisions for. Medical Device Packaging And Labeling.
From klanjwrju.blob.core.windows.net
Fda Medical Device Labeling Guidance at Michael Crawford blog Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Labeling plays an important. Medical Device Packaging And Labeling.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Packaging And Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users,. Medical Device Packaging And Labeling.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Packaging And Labeling Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). •understand the requirements for medical device labeling •review some key labeling provisions for. Medical Device Packaging And Labeling.
From www.packagingconnections.com
Labelling things to know in Pharmaceutical Drugs & Medical Devices Medical Device Packaging And Labeling Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). The ce marking should be affixed to the device or its sterile packaging. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd).. Medical Device Packaging And Labeling.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr).. Medical Device Packaging And Labeling.
From mavink.com
Medical Device Labeling Symbols Medical Device Packaging And Labeling •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. The ce marking should be affixed to the device or its sterile packaging. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labeling plays an important role in. Medical Device Packaging And Labeling.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Packaging And Labeling Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. The ce marking should be affixed to the device or its sterile packaging. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation. Medical Device Packaging And Labeling.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Packaging And Labeling •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Medical Device Packaging And Labeling.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Packaging And Labeling Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. The ce marking should be affixed to the device or its sterile packaging. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Medical devices placed in. Medical Device Packaging And Labeling.
From www.scribd.com
Medical Device Packaging PDF Medical Device Packaging And Labeling Medical Device Packaging And Labeling Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling. Medical Device Packaging And Labeling.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Medical Device Packaging And Labeling Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). •understand the requirements for medical device labeling •review some key labeling provisions for. Medical Device Packaging And Labeling.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling and packaging is key to ensure. Medical Device Packaging And Labeling.
From www.flexo-graphics.com
Medical Device Labeling Archives FlexoGraphics Medical Device Packaging And Labeling Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device. Medical Device Packaging And Labeling.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Packaging And Labeling Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. The ce marking should be affixed to the device or its sterile packaging. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. The purpose of this imdrf guidance is. Medical Device Packaging And Labeling.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Packaging And Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be. Medical Device Packaging And Labeling.
From www.kmedhealth.com
Medical Device Label SymbolsI In Packaging Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties.. Medical Device Packaging And Labeling.
From www.docdroid.net
Medical Device packaging & Labeling Symbols.pdf DocDroid Medical Device Packaging And Labeling Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. •understand the requirements for medical device labeling •review some key labeling provisions for. Medical Device Packaging And Labeling.
From cmo.demetech.us
Packaging & Labeling CMO Demetech Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Labeling and packaging is key. Medical Device Packaging And Labeling.
From www.royallabel.com
The Ultimate Guide to the Medical Device Labeling Process Medical Device Packaging And Labeling Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with. Medical Device Packaging And Labeling.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Packaging And Labeling Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third. Medical Device Packaging And Labeling.
From www.team-consulting.com
Medical device packaging design Team Consulting Medical Device Packaging And Labeling Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in. Medical Device Packaging And Labeling.
From packoi.com
The Role of Packaging in Protecting and Preserving Medical Equipment Medical Device Packaging And Labeling Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including. Medical Device Packaging And Labeling.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Packaging And Labeling Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. The ce marking should be affixed to the device or its sterile packaging. The purpose of this imdrf guidance is. Medical Device Packaging And Labeling.
From influencepackaging.com
Custom Packaging & Medical Device Packaging Influence Packaging Medical Device Packaging And Labeling Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. The ce marking should be affixed to the device or its sterile packaging. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Labeling and packaging is key. Medical Device Packaging And Labeling.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Packaging And Labeling Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including. Medical Device Packaging And Labeling.
From www.ecobliss-pharma.com
Medical Device Packaging Ecobliss Pharmaceutical Packaging Medical Device Packaging And Labeling The ce marking should be affixed to the device or its sterile packaging. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device manufacturers must incorporate in. Medical Device Packaging And Labeling.
From www.ossid.com
Advanced Medical Device & Pharmacuetical Packaging Solutions Ossid Medical Device Packaging And Labeling Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity. Medical Device Packaging And Labeling.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Packaging And Labeling •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling plays an important role in ensuring that medical devices are used safely and effectively for patients, users, and third parties. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to. Medical Device Packaging And Labeling.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Packaging And Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with. Medical Device Packaging And Labeling.
From www.scribd.com
Requirements For Labelling of Medical Devices Mda PDF Medical Medical Device Packaging And Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity. Medical Device Packaging And Labeling.
From www.slideshare.net
Symbols Commonly Used in Medical Device Packaging and Labeling Medical Device Packaging And Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd). •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. Medical Device Packaging And Labeling.