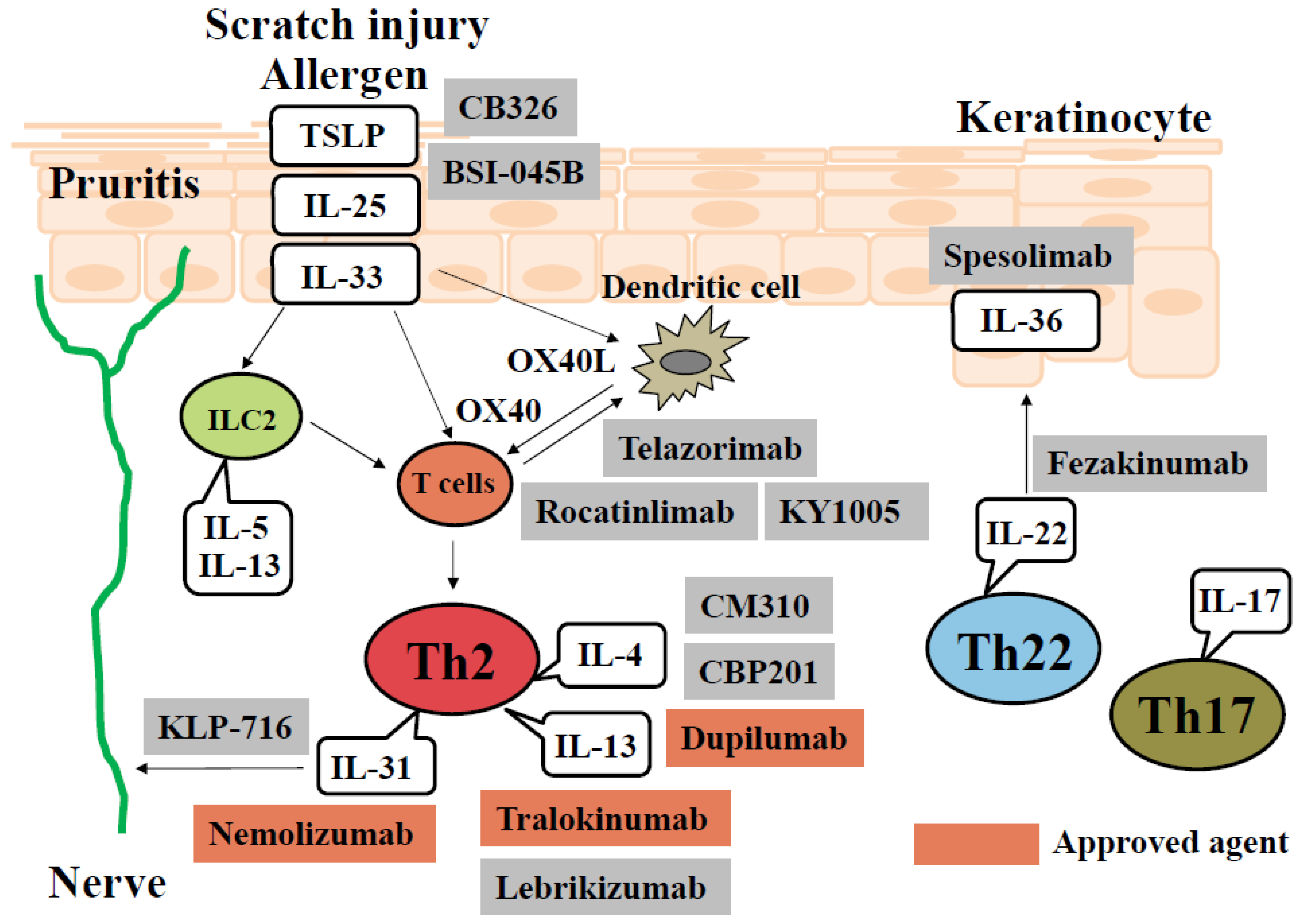
Therapeutic Product Effect Incomplete . Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name drug description; Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and.
from www.mdpi.com
Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name target type; Drug drug name drug description; The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was.
Biomedicines Free FullText Novel Therapeutic Targets for the
Therapeutic Product Effect Incomplete The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Drug drug name drug description; Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the.
From healthynaturalsystems.com
NuTherapy Products Healthy Natural Solutions Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. The most commonly reported aes by preferred term (figure 2a). Therapeutic Product Effect Incomplete.
From scite.ai
Health Canada's Therapeutic Products Directorate has approved a Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name target type; Table 5 lists the substitution from the dexamphetamine product (left column). Therapeutic Product Effect Incomplete.
From www.cevidra.com
Advanced Therapy Medicinal Products Cevidra Therapeutic Product Effect Incomplete Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and.. Therapeutic Product Effect Incomplete.
From www.frontiersin.org
Frontiers How far are the new wave of mRNA drugs from us? mRNA Therapeutic Product Effect Incomplete Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name target type; The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%]. Therapeutic Product Effect Incomplete.
From peakup.edu.vn
Arrhenius concept of Acid And Base Ionisation, Explanation Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a). Therapeutic Product Effect Incomplete.
From www.frontiersin.org
Frontiers Microbiome engineering engineered live biotherapeutic Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the. Therapeutic Product Effect Incomplete.
From chpnz.org.nz
The Therapeutics Product Bill CHPNZ Therapeutic Product Effect Incomplete Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name target type; Drug drug name drug. Therapeutic Product Effect Incomplete.
From microbiologyjournal.org
Therapeutic and Biomedical Potentialities of Terpenoids A Review Therapeutic Product Effect Incomplete Drug drug name target type; Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the.. Therapeutic Product Effect Incomplete.
From www.mdpi.com
Molecules Free FullText Modern Approaches in the Discovery and Therapeutic Product Effect Incomplete Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name target type; Drug drug name drug description; Of note, inappropriate schedule of product administration, drug ineffective, and prescribed. Therapeutic Product Effect Incomplete.
From www.maxqtech.com
Targeting the Therapeutic Effects of Medical Cannabis MaxQ Technologies Therapeutic Product Effect Incomplete Drug drug name target type; Drug drug name drug description; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed. Therapeutic Product Effect Incomplete.
From www.studocu.com
ATI Remediation Forms Therapeutic Procedure STUDENT NAME Therapeutic Product Effect Incomplete The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name target type;. Therapeutic Product Effect Incomplete.
From www.researchgate.net
Diagnostic and therapeutic applications of exosomes. Download Therapeutic Product Effect Incomplete Drug drug name target type; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Drug drug name drug description; Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the.. Therapeutic Product Effect Incomplete.
From dtxalliance.org
Understanding DTx Digital Therapeutics Alliance Therapeutic Product Effect Incomplete Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name drug description; Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,.. Therapeutic Product Effect Incomplete.
From www.mdpi.com
Biomedicines Free FullText Novel Therapeutic Targets for the Therapeutic Product Effect Incomplete Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name drug description; Drug drug name target type; The most commonly reported aes by preferred term (figure 2a) were. Therapeutic Product Effect Incomplete.
From www.mdpi.com
Separations Free FullText An Overview of Herbal Nutraceuticals Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name drug description; Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective. Therapeutic Product Effect Incomplete.
From blog.marketresearch.com
The Power of Digital Therapeutics in Mental Health Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name target type; The most commonly reported aes by preferred term (figure 2a) were. Therapeutic Product Effect Incomplete.
From www.frontiersin.org
Frontiers NextGeneration Approaches Needed to Tackle Antimicrobial Therapeutic Product Effect Incomplete Drug drug name target type; Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Drug drug name drug description;. Therapeutic Product Effect Incomplete.
From microbiomepost.com
Developing a donorbased microbiome therapeutic product MicrobiomePost Therapeutic Product Effect Incomplete Drug drug name drug description; The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,.. Therapeutic Product Effect Incomplete.
From gr-cis.com
Arcturus obtains approval for Phase IIIb Covid vaccine trial in Vietnam Therapeutic Product Effect Incomplete Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name drug description; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name target type;. Therapeutic Product Effect Incomplete.
From jelvix.com
Digital therapeutics benefits, challenges and future prospects Therapeutic Product Effect Incomplete The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column). Therapeutic Product Effect Incomplete.
From www.frontiersin.org
Frontiers Natural Therapeutics in Aid of Treating Alzheimer’s Disease Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name drug description; Drug drug name target type; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. The most commonly reported aes by preferred term (figure 2a) were. Therapeutic Product Effect Incomplete.
From www.slideteam.net
Digital Therapeutic Product Best Practices Digital Therapeutics Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column). Therapeutic Product Effect Incomplete.
From www.picknsave.com
Earth Therapeutics Microwaveable AntiStress Comfort Wrap, 1 ct Pick Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. The most commonly reported aes by preferred term (figure 2a) were related to lack of or. Therapeutic Product Effect Incomplete.
From www.mdpi.com
Nutrients Free FullText Accumulation of Advanced Glycation End Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,.. Therapeutic Product Effect Incomplete.
From organicnz.org.nz
The Therapeutic Products Bill A step forward for natural health Therapeutic Product Effect Incomplete Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the. Therapeutic Product Effect Incomplete.
From www.researchgate.net
Examples of Digital Therapeutics under development or on the market Therapeutic Product Effect Incomplete Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name target type; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name drug description;. Therapeutic Product Effect Incomplete.
From www.mdpi.com
Antioxidants Free FullText State of the Art of Anthocyanins Therapeutic Product Effect Incomplete Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or. Therapeutic Product Effect Incomplete.
From www.slideserve.com
PPT General Principles of Massage PowerPoint Presentation, free Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name target type; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was.. Therapeutic Product Effect Incomplete.
From jpet.aspetjournals.org
Preclinical Characterization of LY3209590, a Novel Weekly Basal Insulin Therapeutic Product Effect Incomplete Drug drug name drug description; Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Drug drug name target type; Table 5 lists the substitution from. Therapeutic Product Effect Incomplete.
From www.studocu.com
Active Learning Template Therapeutic Procedure form ACTIVE LEARNING Therapeutic Product Effect Incomplete Drug drug name target type; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug. Therapeutic Product Effect Incomplete.
From aldric-dd.blogspot.com
Complications Of Blood Transfusion Blood transfusion / Blood Therapeutic Product Effect Incomplete Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name target type; The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Of note, inappropriate schedule. Therapeutic Product Effect Incomplete.
From discover.hubpages.com
Pharmacological Effects, Prodrugs (Definition, Examples) and Sources of Therapeutic Product Effect Incomplete Drug drug name target type; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Drug drug name drug description; Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. The most commonly reported aes by preferred term (figure 2a) were related to lack of or. Therapeutic Product Effect Incomplete.
From www.frontiersin.org
Frontiers Traditional Herbal Medicines, Bioactive Metabolites, and Therapeutic Product Effect Incomplete Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Drug drug name drug description; Table 5 lists the substitution from the dexamphetamine product (left column) that was used in the past, to the suspect drug that was. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. The most commonly reported aes. Therapeutic Product Effect Incomplete.
From drzeydbloodbank.com
Process Therapeutic Apheresis Dr. Zeyd Merenkov Therapeutic Product Effect Incomplete Injection site reactions, diarrhea, pruritus, headache, gastrointestinal disorders, musculoskeletal disorders, nasopharyngitis,. Drug drug name target type; Drug drug name drug description; The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Of note, inappropriate schedule of product administration, drug ineffective, and prescribed. Therapeutic Product Effect Incomplete.
From www.maximizemarketresearch.com
Microbial Therapeutic Products Market (2022 to 2027) Industry analysis Therapeutic Product Effect Incomplete Of note, inappropriate schedule of product administration, drug ineffective, and prescribed underdose were present among the. Drug drug name drug description; Drug drug name target type; The most commonly reported aes by preferred term (figure 2a) were related to lack of or incomplete efficacy of the drug (drug ineffective [18.5%], condition aggravated [13.7%] and. Injection site reactions, diarrhea, pruritus, headache,. Therapeutic Product Effect Incomplete.