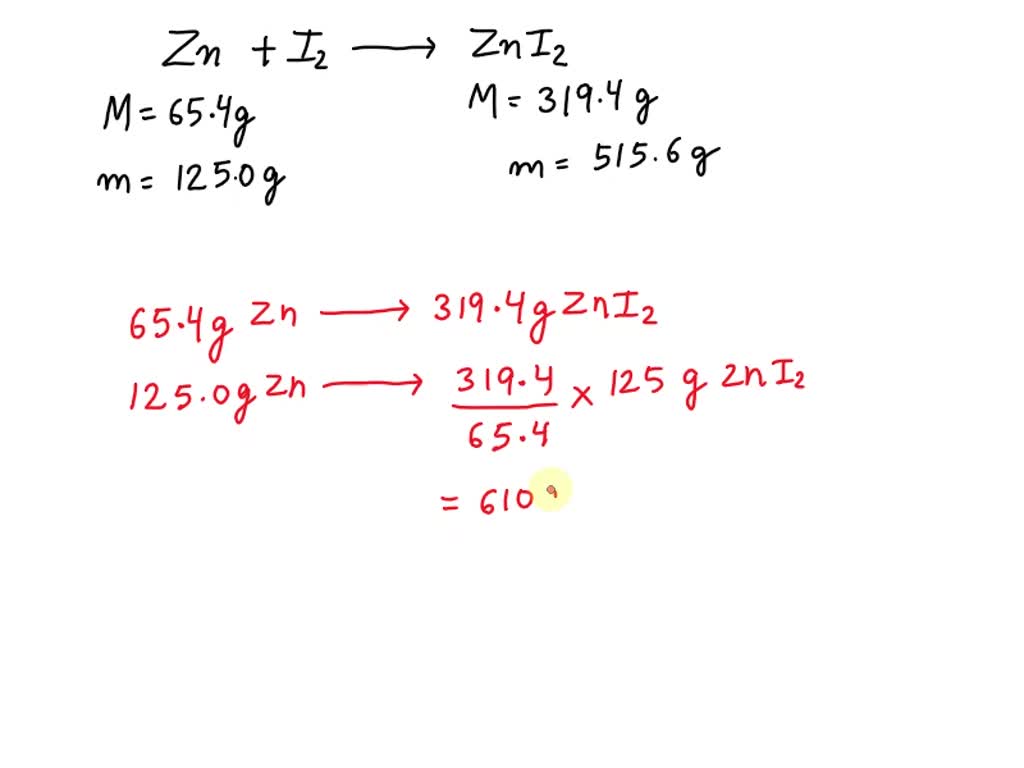Zinc Vapor Formula . Members of a group typically have similar properties and electron configurations in their. Copy sheet of paper on top of another sheet. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Latent heat of fusion of zinc is. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of vaporization of zinc is 115.3 kj/mol. Specific heat of zinc is 0.39 j/g k. A vertical column in the periodic table. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Zinc can be readily cast or.
from www.numerade.com
Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zinc can be readily cast or. Latent heat of fusion of zinc is. Latent heat of vaporization of zinc is 115.3 kj/mol. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Members of a group typically have similar properties and electron configurations in their. A vertical column in the periodic table. Specific heat of zinc is 0.39 j/g k. Copy sheet of paper on top of another sheet.
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a
Zinc Vapor Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). A vertical column in the periodic table. Copy sheet of paper on top of another sheet. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Latent heat of fusion of zinc is. Members of a group typically have similar properties and electron configurations in their. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Specific heat of zinc is 0.39 j/g k. Zinc can be readily cast or. Latent heat of vaporization of zinc is 115.3 kj/mol. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing).
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Zinc Vapor Formula Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Latent heat of fusion of zinc is. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing).. Zinc Vapor Formula.
From www.dreamstime.com
3D Image of Zinc Sulfate Skeletal Formula Stock Illustration Zinc Vapor Formula This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Copy sheet of paper on top of another sheet. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Latent heat of fusion of zinc is. In case of liquid to. Zinc Vapor Formula.
From armsingle10.pythonanywhere.com
Peerless Molecular Formula Of Rust Write The Balanced Equation Zinc Vapor Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zinc can be readily cast or. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Members of a group typically have similar properties and electron configurations in their. Specific heat of zinc is. Zinc Vapor Formula.
From cartoondealer.com
Zinc Periodic Table Of The Elements Vector Illustration CartoonDealer Zinc Vapor Formula Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of vaporization of zinc is 115.3 kj/mol. Members of a group typically have similar properties and electron configurations in their. A vertical column. Zinc Vapor Formula.
From www.alamy.es
El óxido de zinc es una fórmula química molecular. Infografías sobre Zinc Vapor Formula In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of fusion of zinc is. Copy sheet of paper on top of another sheet. Zinc can be readily cast or. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Latent heat. Zinc Vapor Formula.
From www.shutterstock.com
Zinc Oxide Chemical Formula Inside Green Stock Vector (Royalty Free Zinc Vapor Formula This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Copy sheet of paper on top of another sheet. Specific heat of zinc is 0.39 j/g k. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). A vertical column in. Zinc Vapor Formula.
From www.911metallurgist.com
Zinc Vapor Condensation Zinc Vapor Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Specific heat of zinc is 0.39 j/g k. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Latent heat of vaporization of zinc is 115.3 kj/mol. Zinc can be readily. Zinc Vapor Formula.
From www.chegg.com
Solved REPORT SHEET Chemical Formulas A, Zinc Chloride 1. Zinc Vapor Formula Members of a group typically have similar properties and electron configurations in their. Latent heat of vaporization of zinc is 115.3 kj/mol. A vertical column in the periodic table. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Specific heat of zinc is 0.39 j/g k. Zinc oxide obtained from roasting. Zinc Vapor Formula.
From www.dreamstime.com
Zinc Sulfate is a Molecular Chemical Formula. Zinc Infographics. Vector Zinc Vapor Formula Latent heat of vaporization of zinc is 115.3 kj/mol. Zinc can be readily cast or. Latent heat of fusion of zinc is. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Copy sheet of paper on top of another sheet. Specific heat of zinc is 0.39 j/g k. In case of. Zinc Vapor Formula.
From www.dreamstime.com
Zinc Glycinate is a Molecular Chemical Formula. Zinc Infographics Zinc Vapor Formula Members of a group typically have similar properties and electron configurations in their. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. A vertical column in the periodic table. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). In. Zinc Vapor Formula.
From www.researchgate.net
Equilibrium zinc vapor pressure in BF off gas at different temperatures Zinc Vapor Formula Zinc can be readily cast or. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Members of a group typically have similar properties and electron configurations in their. Latent heat of vaporization of zinc is 115.3 kj/mol. This table gives coefficients in an equation for the vapor pressure of metallic elements. Zinc Vapor Formula.
From cartoondealer.com
Zinc Picolinate Molecular Chemical Formula. Zinc Infographics. Vector Zinc Vapor Formula Copy sheet of paper on top of another sheet. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of vaporization of zinc is 115.3 kj/mol. Specific heat of zinc is 0.39 j/g k. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like. Zinc Vapor Formula.
From www.alamy.es
El zincccinado es una fórmula química molecular. Infografías sobre Zinc Vapor Formula Members of a group typically have similar properties and electron configurations in their. Zinc can be readily cast or. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of fusion of zinc. Zinc Vapor Formula.
From www.alamy.es
Vectores de vectores de sulfato fotografías e imágenes de alta Zinc Vapor Formula Zinc can be readily cast or. Latent heat of fusion of zinc is. Latent heat of vaporization of zinc is 115.3 kj/mol. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain.. Zinc Vapor Formula.
From www.alamy.com
Zinc oxide is a molecular chemical formula. Zinc infographics. Vector Zinc Vapor Formula In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Copy sheet of paper on top of another sheet. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Members of a group typically have similar properties and electron configurations in. Zinc Vapor Formula.
From www.youtube.com
How to Write the Formula for Zinc oxide (ZnO) YouTube Zinc Vapor Formula Members of a group typically have similar properties and electron configurations in their. Zinc can be readily cast or. A vertical column in the periodic table. Latent heat of fusion of zinc is. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. In case of liquid to gas phase change, this amount. Zinc Vapor Formula.
From kunduz.com
[ANSWERED] 1 Vapor pressure equation for liquid zinc is given by the Zinc Vapor Formula Specific heat of zinc is 0.39 j/g k. Latent heat of fusion of zinc is. Copy sheet of paper on top of another sheet. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. A vertical column in the periodic table. In case of liquid to gas phase change, this amount of energy. Zinc Vapor Formula.
From www.pw.live
Zinc Phosphate Formula, Properties And Uses Zinc Vapor Formula This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Zinc can be readily cast or. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Copy sheet of paper on top of another sheet. Latent heat of fusion of zinc is.. Zinc Vapor Formula.
From cartoondealer.com
Zinc Sulfate Is A Molecular Chemical Formula. Zinc Infographics. Vector Zinc Vapor Formula Latent heat of vaporization of zinc is 115.3 kj/mol. Members of a group typically have similar properties and electron configurations in their. Copy sheet of paper on top of another sheet. Latent heat of fusion of zinc is. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. In. Zinc Vapor Formula.
From www.congress-intercultural.eu
Zinc Oxide Formula Properties Manufacturing Citra, 43 OFF Zinc Vapor Formula This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Members of a group typically have similar properties and electron configurations in their. Zinc can be readily cast or. Latent heat. Zinc Vapor Formula.
From www.numerade.com
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a Zinc Vapor Formula Specific heat of zinc is 0.39 j/g k. Latent heat of fusion of zinc is. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Zinc can be readily cast or. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Zinc. Zinc Vapor Formula.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Zinc Vapor Formula This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. A vertical column in the periodic table. Zinc can be readily cast or. In case of liquid to gas phase change, this. Zinc Vapor Formula.
From klabxtjsm.blob.core.windows.net
Potassium Hydroxide And Zinc Equation at James Roden blog Zinc Vapor Formula Members of a group typically have similar properties and electron configurations in their. Latent heat of fusion of zinc is. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of vaporization of zinc is 115.3 kj/mol. Specific heat of zinc is 0.39 j/g k. Zinc is reasonably resistant to. Zinc Vapor Formula.
From es.dreamstime.com
El Sulfato De Zinc Es Una Fórmula Química Molecular. Infografía De Zinc Zinc Vapor Formula Latent heat of fusion of zinc is. Copy sheet of paper on top of another sheet. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Latent heat of vaporization of zinc is 115.3 kj/mol. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high. Zinc Vapor Formula.
From tia-jolpblogramsey.blogspot.com
Zinc Carbonate Sulfuric Acid Zinc Vapor Formula Members of a group typically have similar properties and electron configurations in their. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Copy sheet of paper on top of another sheet. Zinc can be readily cast or. This table gives coefficients in an equation for the vapor pressure of metallic elements. Zinc Vapor Formula.
From www.alamy.com
Zinc sulfate is a molecular chemical formula. Zinc infographics. Vector Zinc Vapor Formula Latent heat of vaporization of zinc is 115.3 kj/mol. Specific heat of zinc is 0.39 j/g k. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of fusion of zinc is. This. Zinc Vapor Formula.
From www.numerade.com
SOLVED Consider the reaction of zinc metal with hydrochloric acid, HCl Zinc Vapor Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Members of a group typically have similar properties and electron configurations in their. Specific heat of zinc is 0.39 j/g k. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state.. Zinc Vapor Formula.
From www.chegg.com
Solved The vapor pressure of solid NaF varies with Zinc Vapor Formula Zinc can be readily cast or. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent heat of fusion of zinc is. Copy sheet of paper on top of another. Zinc Vapor Formula.
From www.dreamstime.com
Zinc Oxide is a Molecular Chemical Formula. Zinc Infographics. Vector Zinc Vapor Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Zinc can be readily cast or. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Latent. Zinc Vapor Formula.
From www.alamy.es
Compuestos moleculares de zinc. La fórmula química es picolinato Zinc Vapor Formula Latent heat of fusion of zinc is. Specific heat of zinc is 0.39 j/g k. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). A vertical column in the periodic table. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. This table. Zinc Vapor Formula.
From www.youtube.com
How to Balance ZnO + C = Zn (Vapor) + CO YouTube Zinc Vapor Formula This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Latent heat of fusion of zinc is. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Latent heat of vaporization of zinc is 115.3 kj/mol. Specific heat of zinc is. Zinc Vapor Formula.
From www.alamy.com
Zinc picolinate molecular chemical formula. Zinc infographics. Vector Zinc Vapor Formula Copy sheet of paper on top of another sheet. Latent heat of vaporization of zinc is 115.3 kj/mol. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zinc can be readily cast or. Latent heat of fusion of zinc is. Specific heat of zinc is 0.39 j/g k. A vertical column. Zinc Vapor Formula.
From www.researchgate.net
Zinc vapor in BF bosh dissolves into molten iron. Download Scientific Zinc Vapor Formula Specific heat of zinc is 0.39 j/g k. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. This table gives coefficients in an equation for the vapor pressure of metallic elements in both the solid and liquid state. Copy sheet of paper on top of another sheet. A vertical column in. Zinc Vapor Formula.
From www.numerade.com
SOLVED A 20.00 g mixture of magnesium and zinc metal reacting with Zinc Vapor Formula In case of liquid to gas phase change, this amount of energy is known as the enthalpy of. Zinc can be readily cast or. Zinc oxide obtained from roasting is reduced with carbon or carbon monoxide at high temperatures to obtain. Latent heat of vaporization of zinc is 115.3 kj/mol. Latent heat of fusion of zinc is. Specific heat of. Zinc Vapor Formula.
From www.numerade.com
SOLVED The normal boiling point of zinc is 1180 K. Calculate its vapor Zinc Vapor Formula A vertical column in the periodic table. Specific heat of zinc is 0.39 j/g k. Zinc can be readily cast or. Members of a group typically have similar properties and electron configurations in their. Latent heat of vaporization of zinc is 115.3 kj/mol. In case of liquid to gas phase change, this amount of energy is known as the enthalpy. Zinc Vapor Formula.